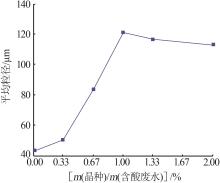
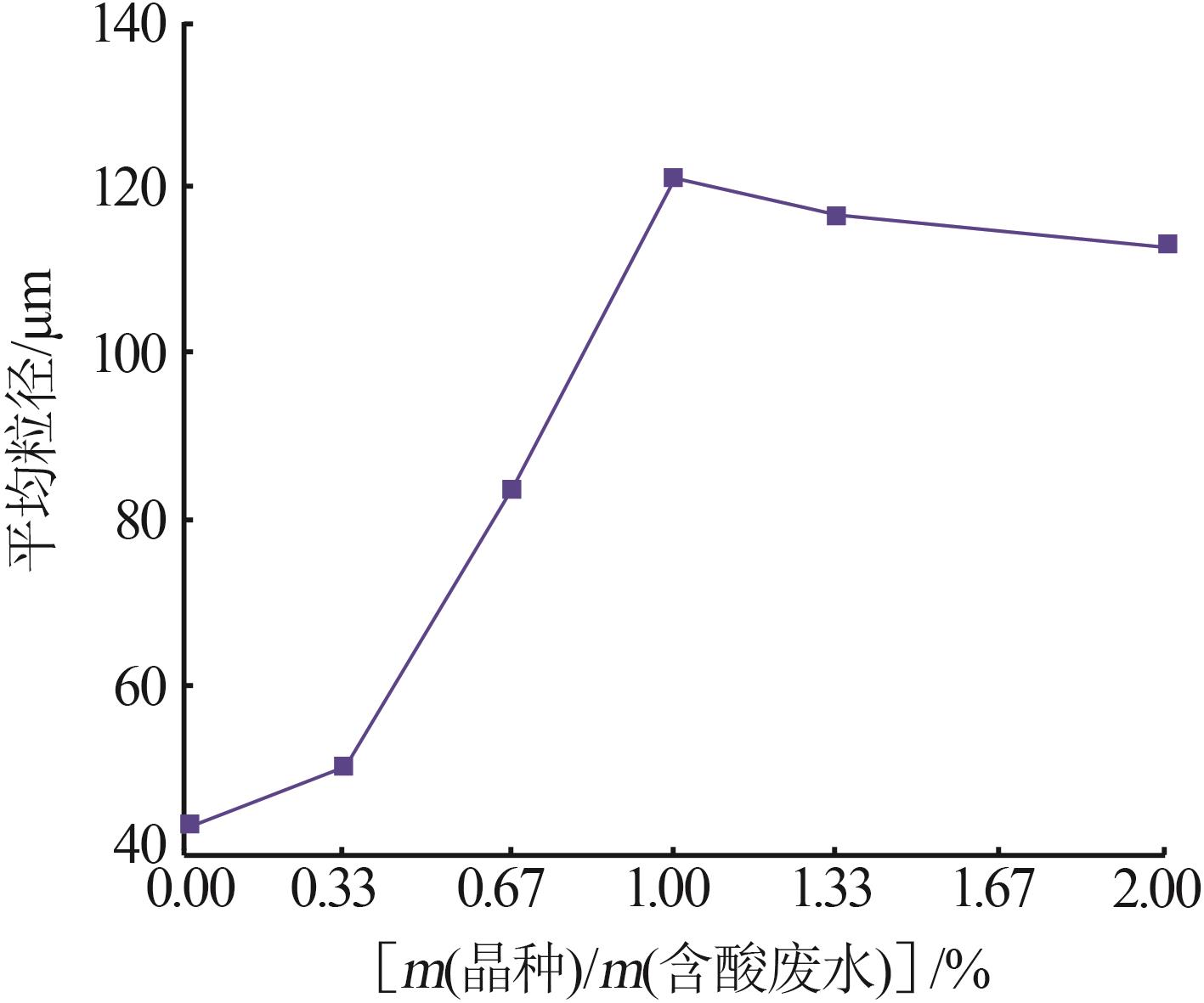
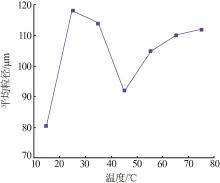
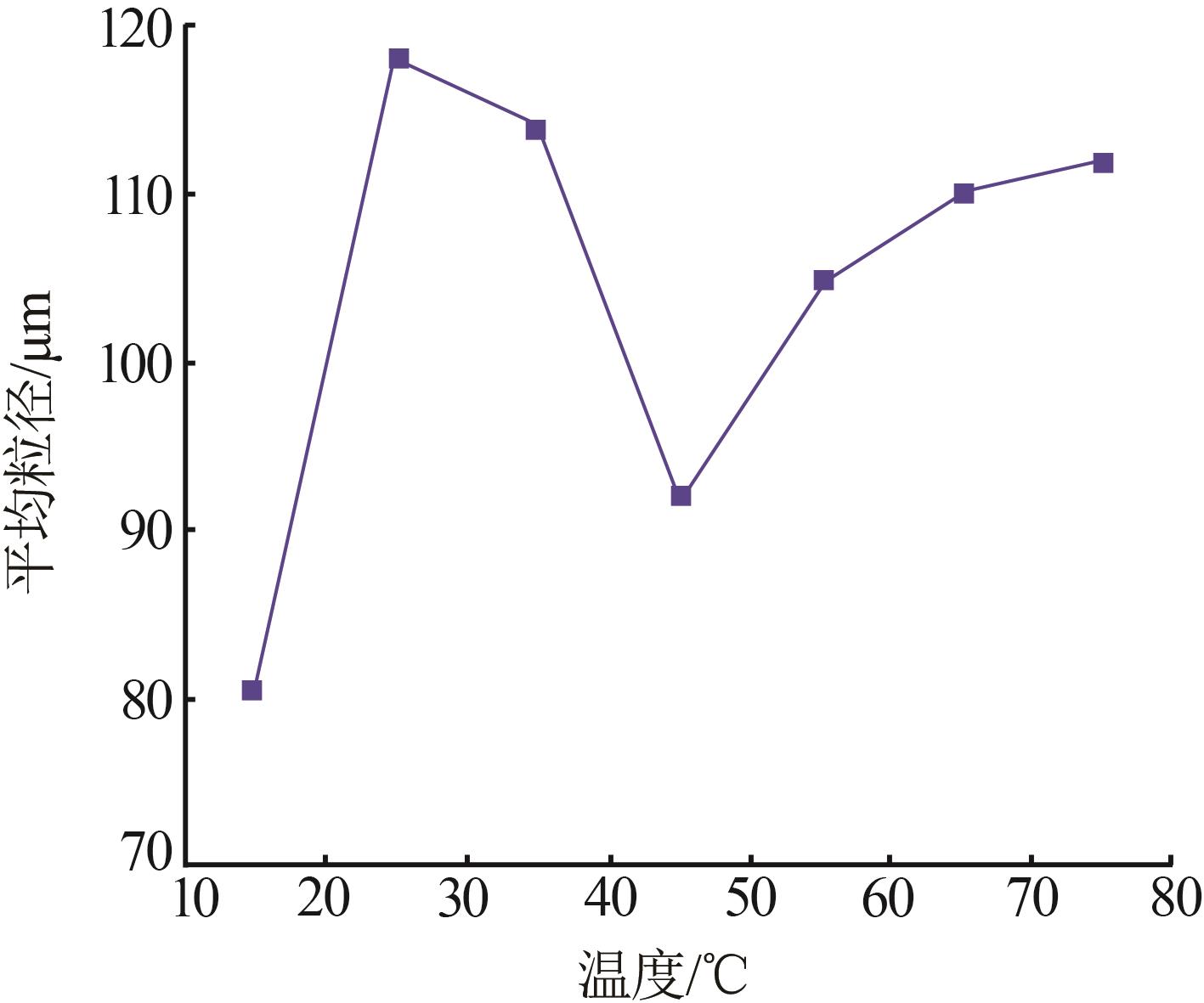
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (11): 78-85.doi: 10.19964/j.issn.1006-4990.2023-0136
• Industrial Techniques • Previous Articles Next Articles
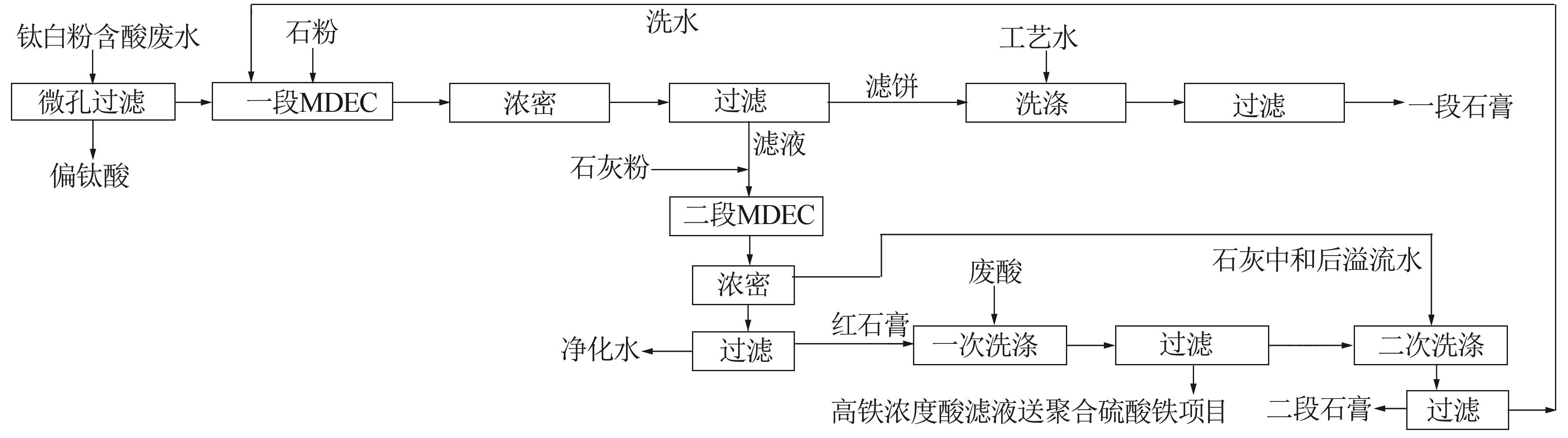
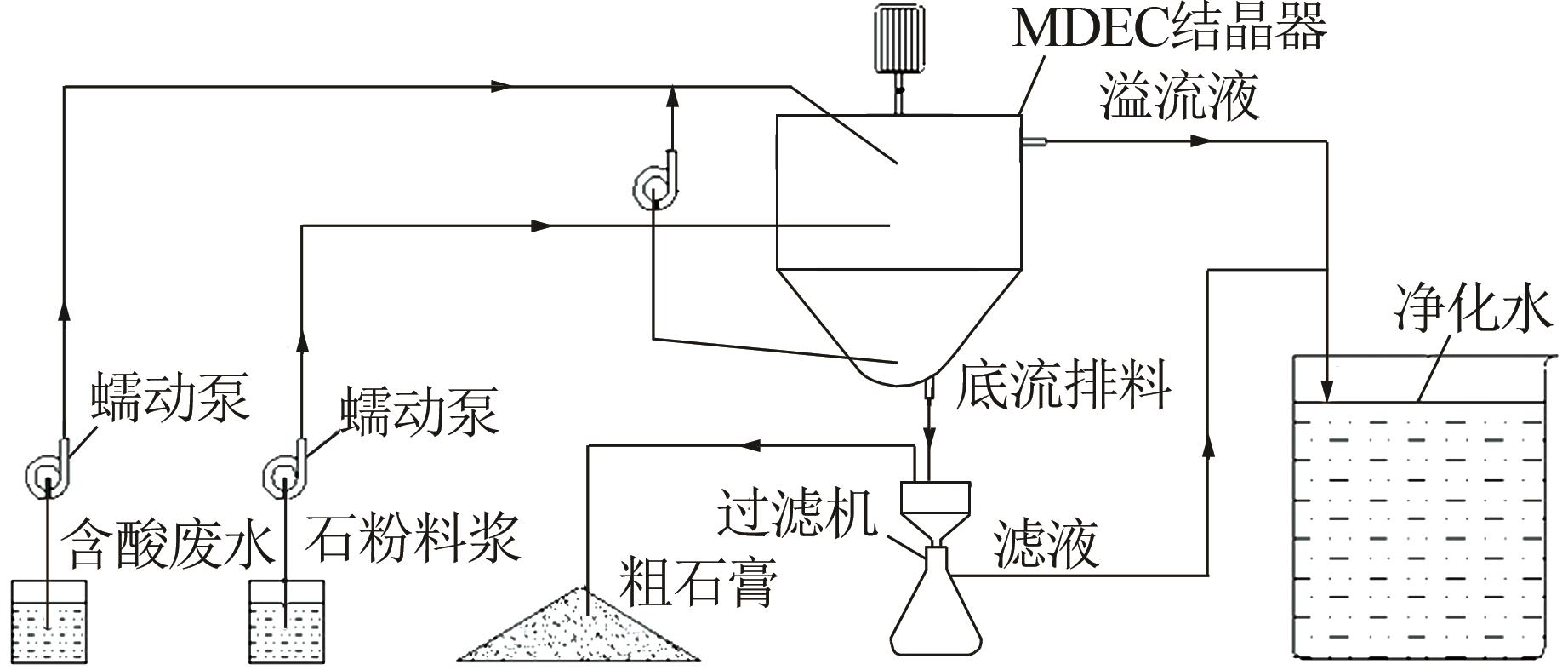
Research on mechanical drive enhancement crystallization process for titanium gypsum
WEI Kang1( ), ZHANG Hao1, GAN Shunpeng2
), ZHANG Hao1, GAN Shunpeng2
- 1.Guangxi Bluestar Dahua Chemical Co.,Ltd.,Baise 533001,China
2.Zhonglan Changhua Engineering Technology Co.,Ltd.,Changsha 410116,China
-
Received:2023-03-13Online:2023-11-10Published:2023-11-16
CLC Number:
Cite this article
WEI Kang, ZHANG Hao, GAN Shunpeng. Research on mechanical drive enhancement crystallization process for titanium gypsum[J]. Inorganic Chemicals Industry, 2023, 55(11): 78-85.
share this article
Table 1
Chemical analysis results of acid-containing wastewater"
| 样品 | w(Ti4+)/% | w(Fe)/% | w(Mn2+)/% | w(Zn2+)/% | w(Ca2+)/% | w(Mg2+)/% | w(SO42-)/% | pH |
|---|---|---|---|---|---|---|---|---|
| 1-水样 | 0.009 3 | 0.122 7 | 0.005 8 | 0.002 2 | 0.006 3 | 0.008 4 | 2.536 | 1.01 |
| 2-水样 | 0.000 2 | 0.354 4 | 0.000 7 | 0.000 1 | 0.021 9 | 0.002 0 | 2.796 | 0.78 |
| 3-水样 | 0.026 6 | 0.418 7 | 0.017 6 | 0.002 2 | 0.016 9 | 0.023 0 | 3.041 | 0.45 |
| 废酸(w | 0.006 0 | 0.389 4 | 0.192 0 | 0.092 0 | 0.003 2 | 0.002 9 | 21.370 | — |
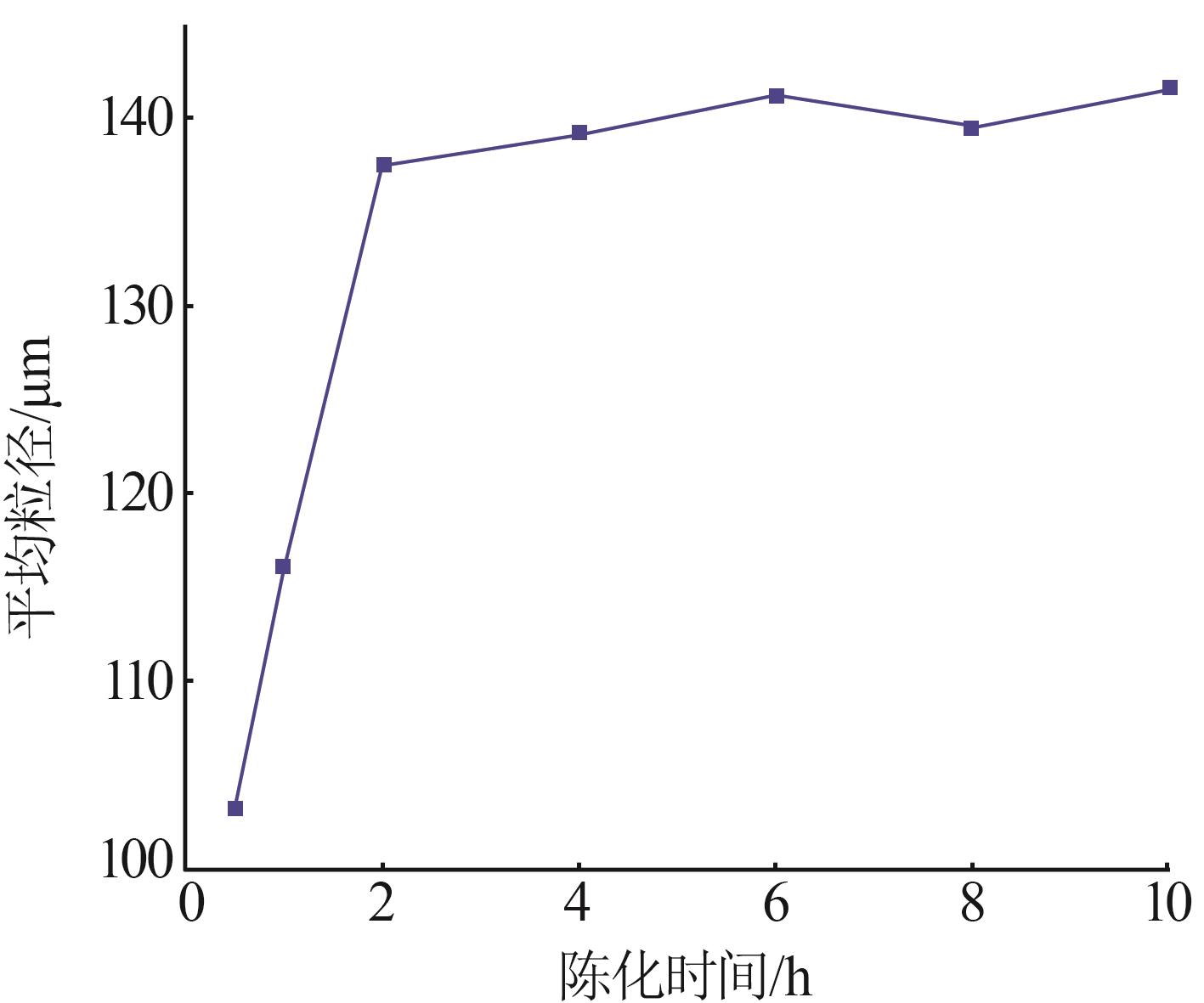
Table 4
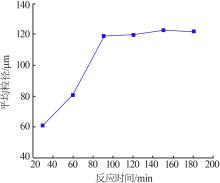
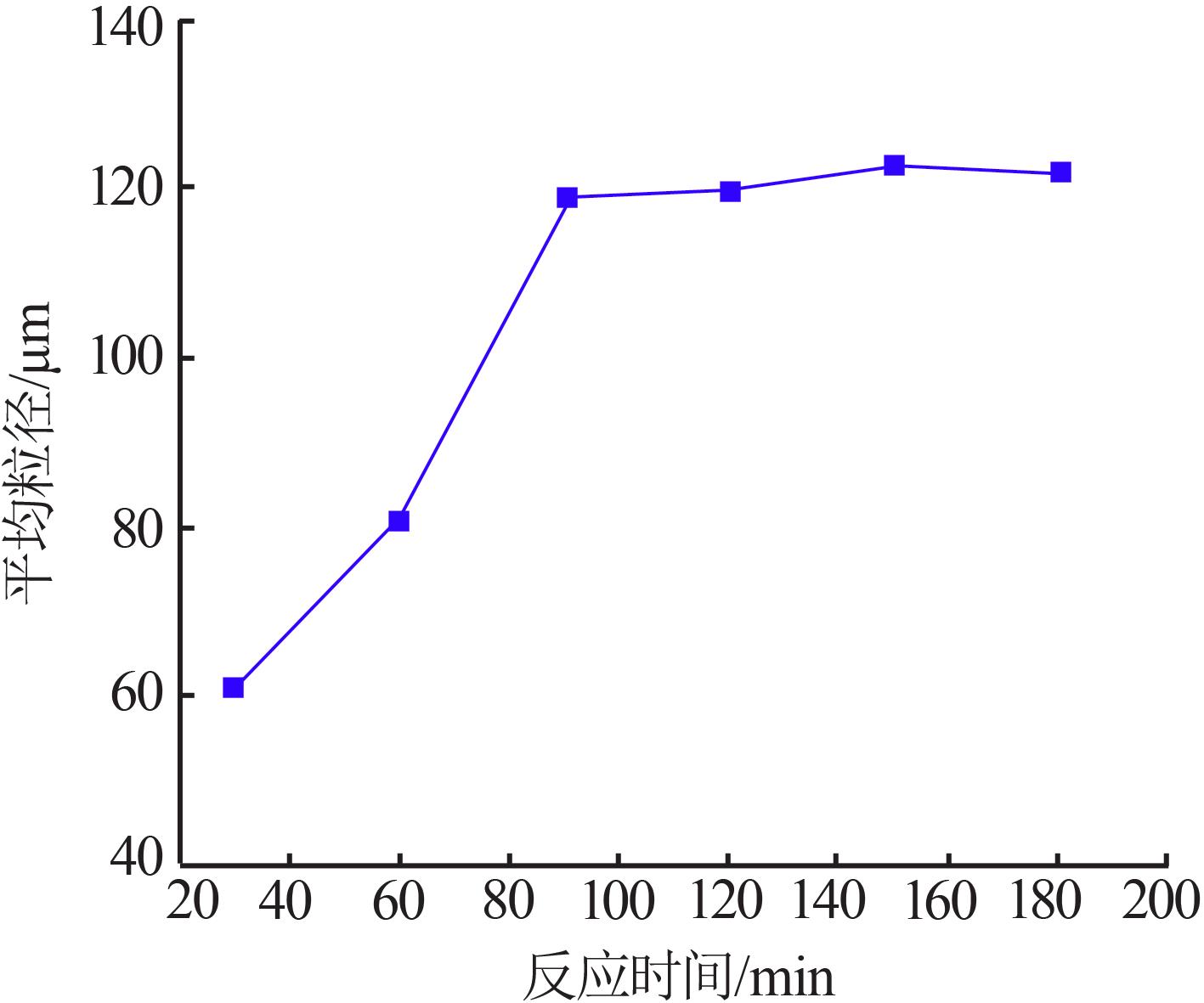
Effect of different treatment capacity on particle size of gypsum"
| 水样 | 处理量/ (L·h-1) | 样品 名称 | 石膏平均粒径/um | w(Fe)/ % | w(Ca2+)/ % | w(SO42-)/% |
|---|---|---|---|---|---|---|
| 2-水样 | 1.67 | 2-水样 | 0.358 | 0.023 | 2.858 | |
| 一段石膏 | 100.9 | 0.265 | 19.120 | 47.880 | ||
| 一段滤液 | 0.274 | 0.074 | 0.824 | |||
| 2-水样 | 3.33 | 2-水样 | 0.358 | 0.023 | 2.858 | |
| 一段石膏 | 130.1 | 0.217 | 18.830 | 48.220 | ||
| 一段滤液 | 0.322 | 0.067 | 0.960 | |||
| 2-水样 | 10 | 2-水样 | 0.358 | 0.023 | 2.858 | |
| 一段石膏 | 151.7 | 0.179 | 18.410 | 42.510 | ||
| 一段滤液 | 0.115 | 0.055 | 0.766 |
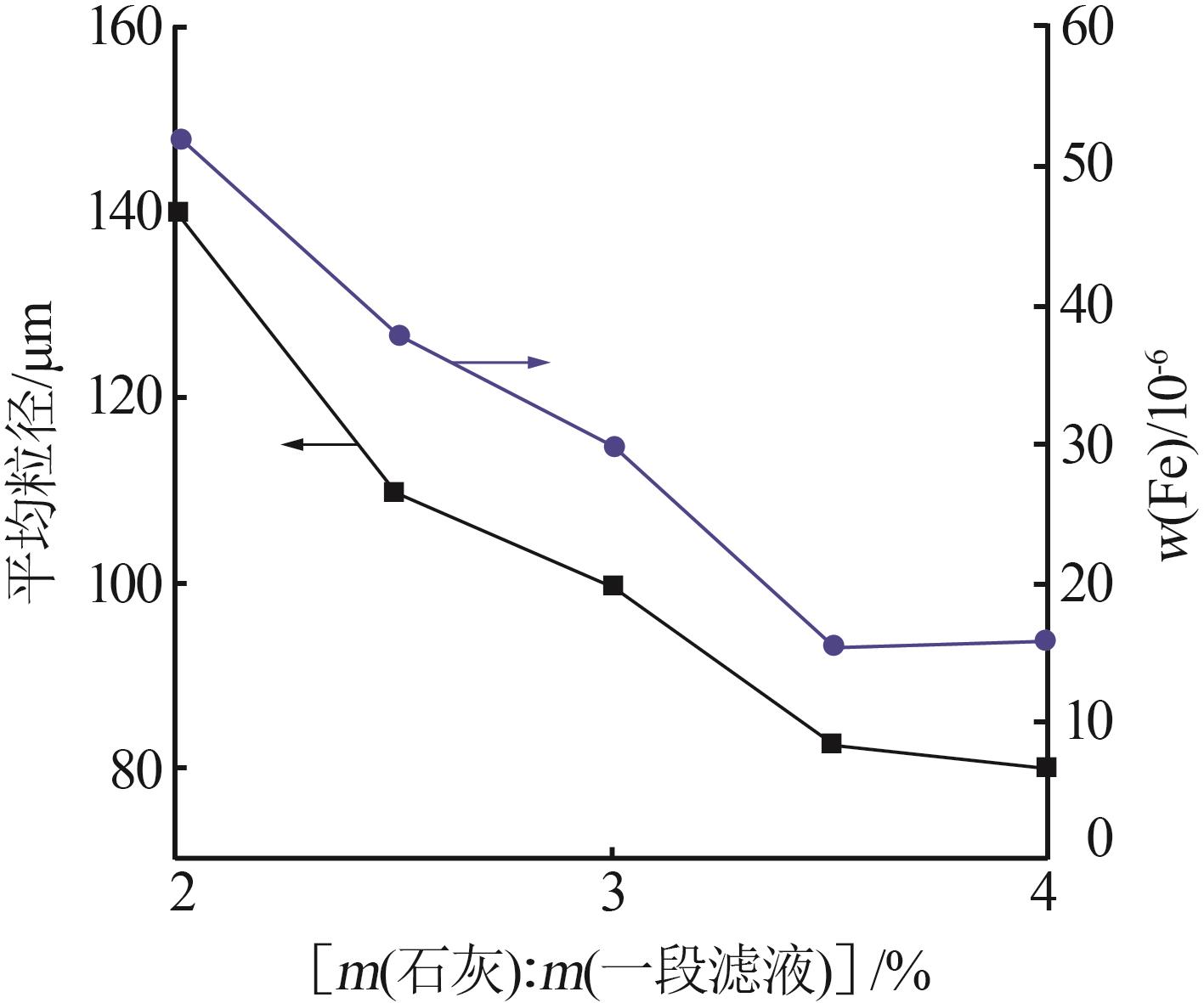
Table 5
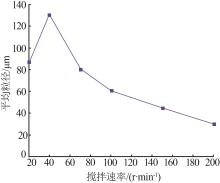
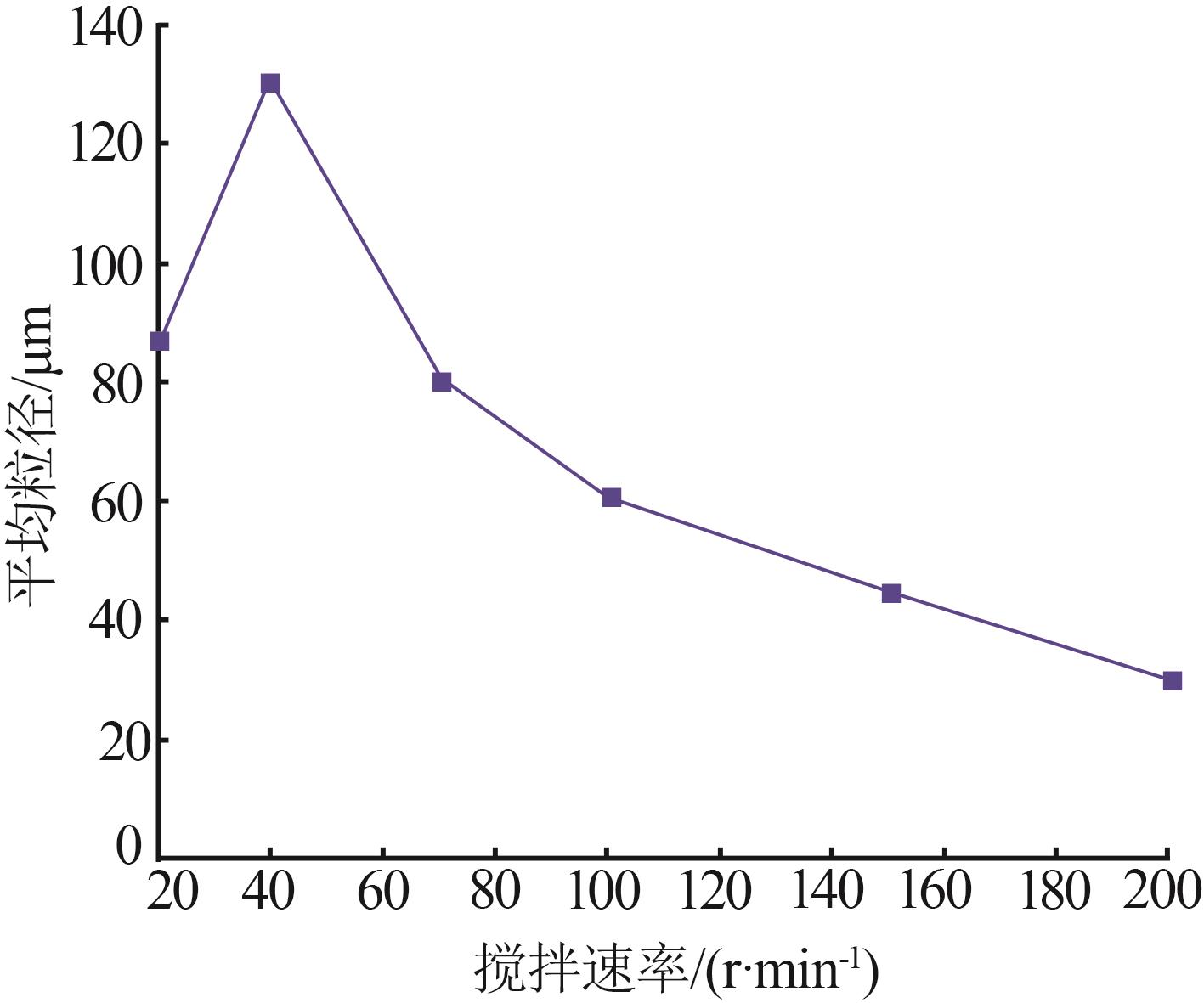
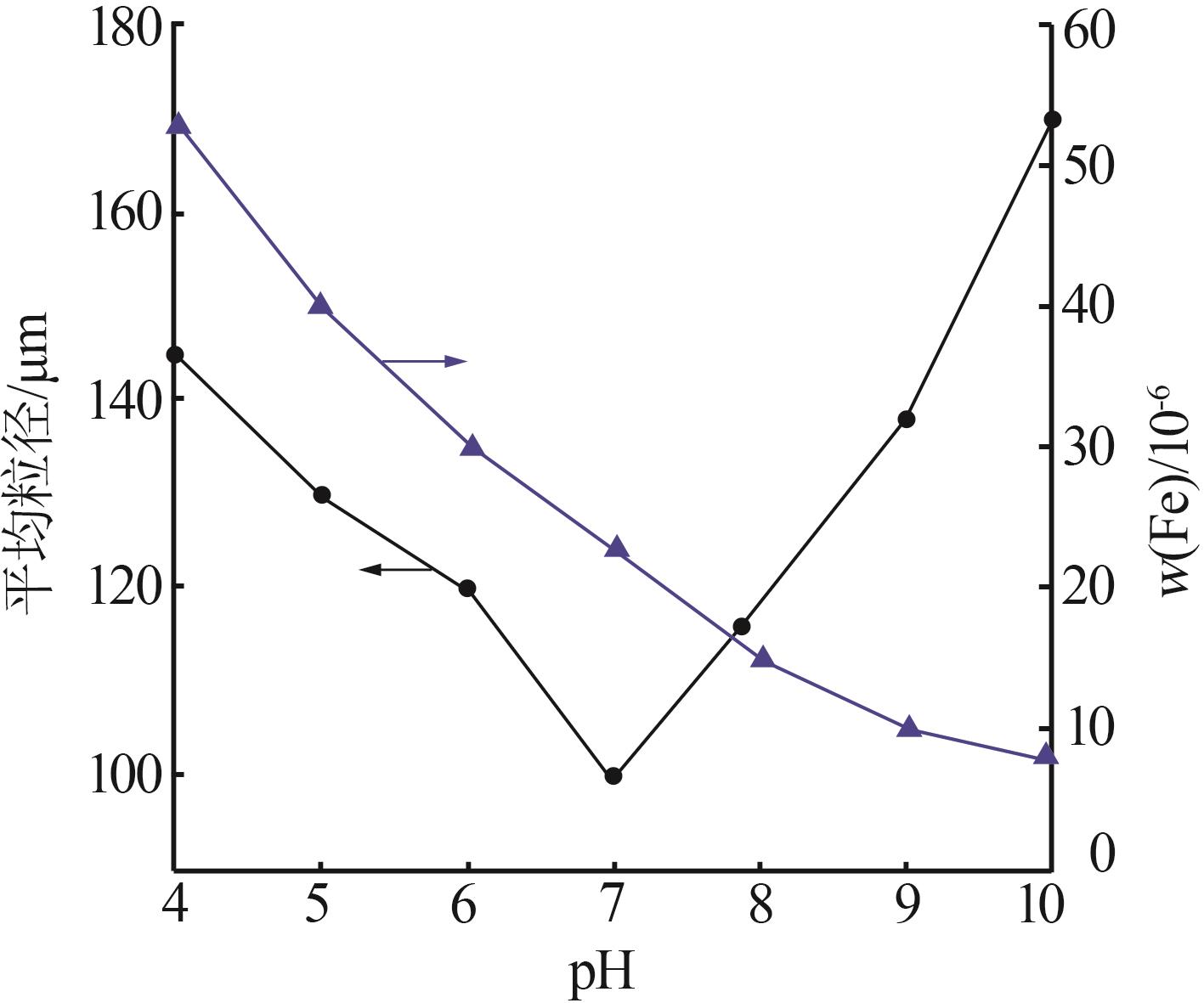
Effect of different pH in crystal regionon particle size of gypsum"
| 水样 | pH | 样品名称 | 石膏平均 粒径/μm | w(Fe)/ % | w (Ca2+)/% | w (SO42-)/% |
|---|---|---|---|---|---|---|
| 3-水样 | 2.5 | 3-水样 | 0.419 | 0.017 | 3.041 | |
| 一段石膏 | 107.4 | 0.068 | 17.760 | 44.53 | ||
| 一段滤液 | 0.171 | 0.061 | 0.957 | |||
| 3-水样 | 3.5 | 3-水样 | 0.419 | 0.017 | 3.041 | |
| 一段石膏 | 137.5 | 0.116 | 20.050 | 49.05 | ||
| 一段滤液 | 0.172 | 0.063 | 0.82 5 | |||
| 3-水样 | 4.5 | 3-水样 | 0.419 | 0.017 | 3.041 | |
| 一段石膏 | 120.2 | 0.080 | 19.170 | 44.19 | ||
| 一段滤液 | 0.168 | 0.065 | 0.875 | |||
| 3-水样 | 5.5 | 3-水样 | 0.419 | 0.017 | 3.041 | |
| 一段粗石膏 | 107.6 | 0.113 | 19.120 | 47.60 | ||
| 一段滤液 | 0.168 | 0.053 | 0.827 |
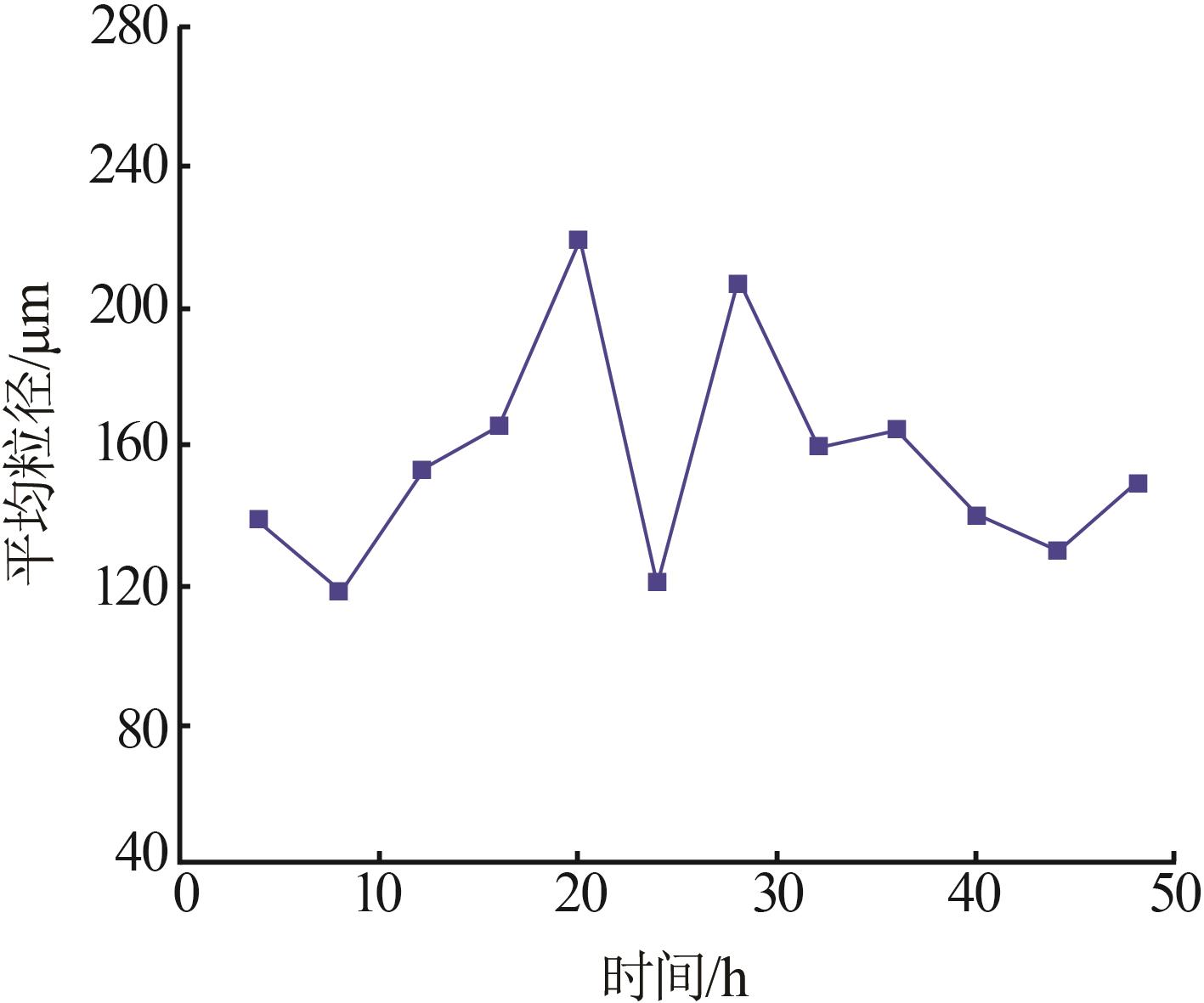
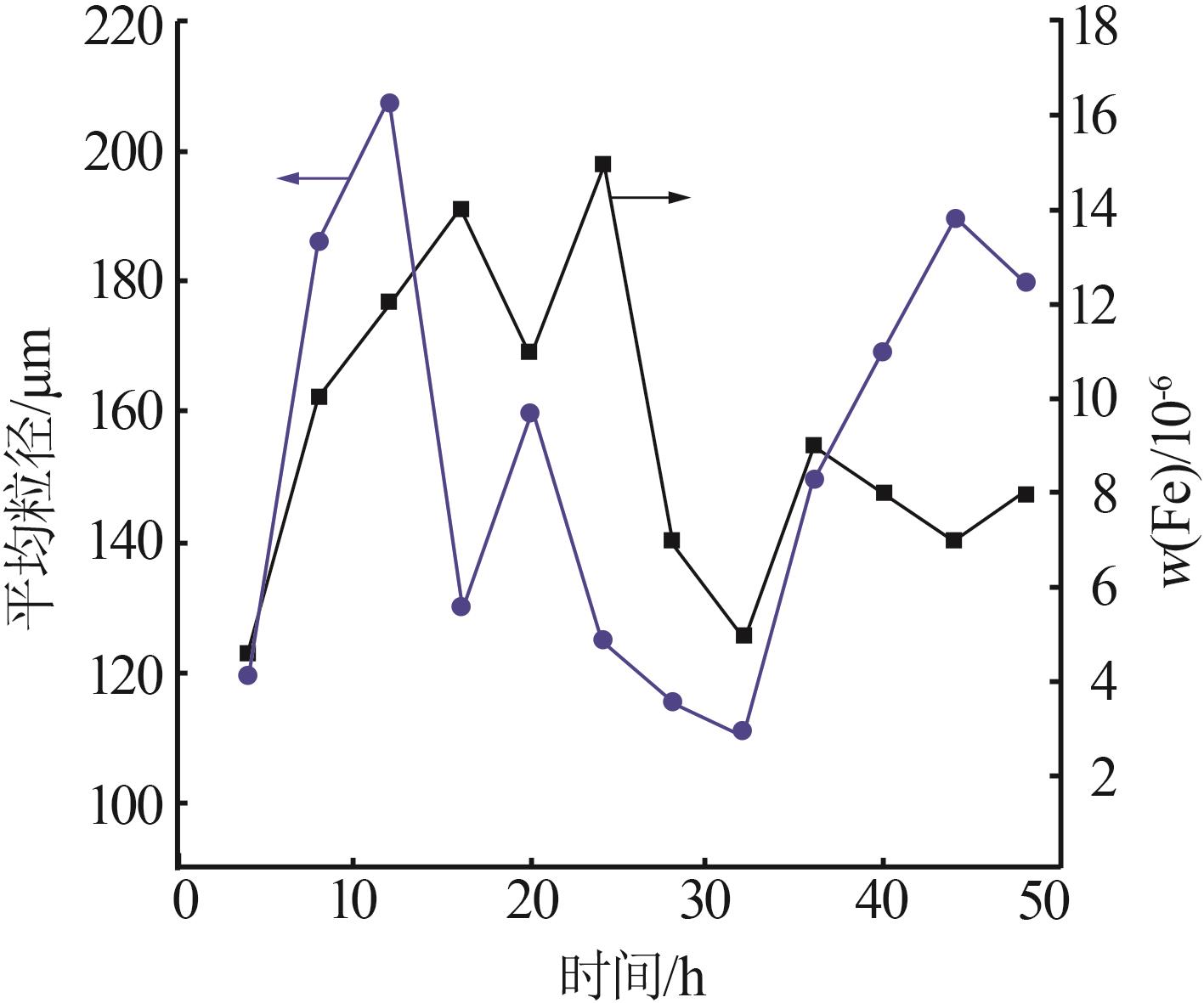
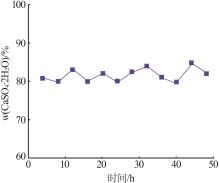
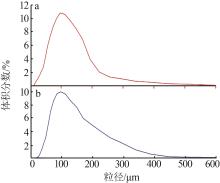
Table 6
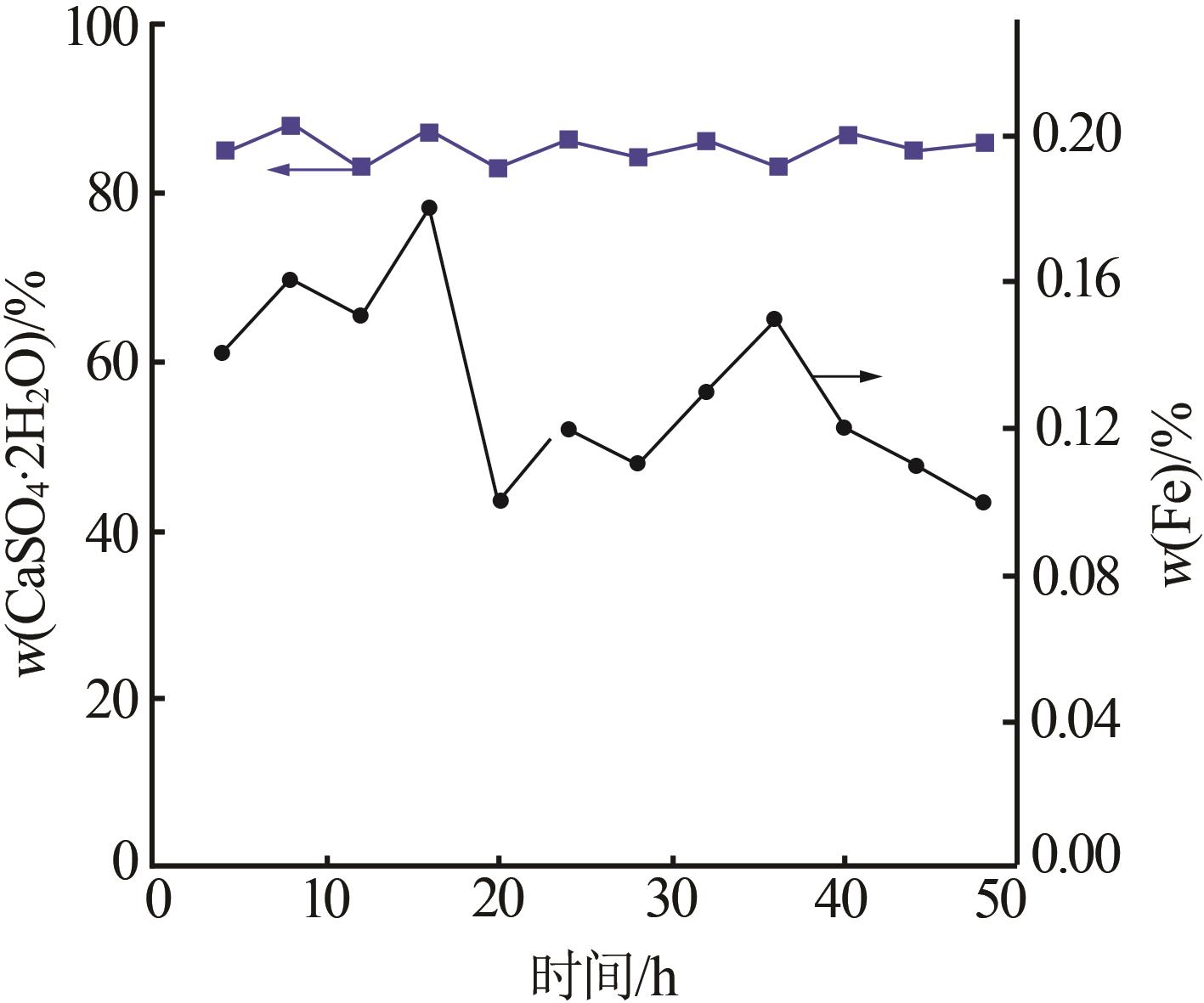
Analysis results of first-stage andsecond-stage gypsum"
| 序号 | 石膏 | w(CaSO4· 2H2O)/% | 平均粒径/ μm | 白度/% |
|---|---|---|---|---|
| 1 | 一段石膏 | 87.00 | 106.60 | 86.33 |
| 2 | 87.00 | 111.30 | 86.15 | |
| 3 | 88.80 | 116.00 | 88.21 | |
| 4 | 87.48 | 113.20 | 84.17 | |
| 5 | 89.26 | 110.10 | 87.87 | |
| 6 | 90.21 | 103.00 | 91.55 | |
| 7 | 88.19 | 115.60 | 89.62 | |
| 8 | 89.53 | 112.20 | 90.41 | |
| 1 | 二段石膏 | 84.67 | 123.60 | 80.00 |
| 2 | 81.48 | 136.00 | 82.15 | |
| 3 | 85.96 | 116.70 | 82.41 | |
| 4 | 80.00 | 100.00 | 87.92 | |
| 5 | 83.92 | 114.70 | 86.76 | |
| 6 | 86.47 | 120.20 | 80.09 | |
| 7 | 83.64 | 122.20 | 81.82 | |
| 8 | 82.05 | 112.70 | 80.00 |
Table 7
Chemical compositions of first-stage and second-stage gypsum"
| 项目 | 气味 | w(CaSO4·2H2O) (干基)/% | w(附着 水)(湿基)/% | w(Fe2O3)(干基)/ % | w(MgO) (干基)/ % | w(Na2O)(干基)/ % | w(K2O) (干基)/ % | w(Cl-) (干基)/ % | pH (干基) | w(其他)/ % | 放射性 |
|---|---|---|---|---|---|---|---|---|---|---|---|
JC/T 2625— 2021一级品 | 无异味 | ≥85.00 | ≤20.00 | ≤8.00 | ≤0.20 | ≤0.06 | ≤0.06 | ≤0.02 | 5.0~9.0 | 其他杂质含量控制由供需双方协商确定 | 符合GB 6566—2010《建筑材料放射性核素限量》标准 |
| 无异味 | |||||||||||
| 一段石膏 | 87.21 | 11.79 | 1.01 | 0.14 | 0.03 | 0.02 | — | 6.95 | |||
| 无异味 | |||||||||||
| 二段石膏 | 81.53 | 9.23 | 1.52 | 0.13 | 0.03 | 0.02 | — | 7.03 |
| 1 | 龚家竹.钛白粉生产技术[M].北京:化学工业出版社,2022. |
| 2 | GÁZQUEZ M J, BOLÍVAR J P, GARCÍA-TENORIO R,et al.Physicochemical characterization of raw materials and co-products from the titanium dioxide industry[J].Journal of Hazardous Materials,2009,166(2/3):1429-1440. |
| 3 | GÁZQUEZ M J, BOLÍVAR J P, GARCIA-TENORIO R,et al.A review of the production cycle of titanium dioxide pigment[J].Materials Sciences and Applications,2014,5(7):441-458. |
| 4 | 纪利春.硫酸法钛白副产物绿矾的资源化利用现状[J].无机盐工业,2020,52(5):11-17. |
| JI Lichun.Present status of resource utilization of by-product green vitriol from titanium dioxide production by sulfuric acid method[J].Inorganic Chemicals Industry,2020,52(5):11-17. | |
| 5 | 陶厚东,马征程,齐飞.钛白废酸高效高值利用工业实践[J].中国资源综合利用,2019,37(5):79-81. |
| TAO Houdong, MA Zhengcheng, QI Fei.Industrial practice of high efficiency and high value utilization of titanium white waste acid[J].China Resources Comprehensive Utilization,2019,37(5):79-81. | |
| 6 | 高广言,高利坤,饶兵,等.硫酸法钛白废酸资源化利用现状及展望[J].钢铁钒钛,2021,42(5):99-108. |
| GAO Guangyan, GAO Likun, RAO Bing,et al.Current situation of resource utilization of waste acid from titanium dioxide production[J].Iron Steel Vanadium Titanium,2021,42(5):99-108. | |
| 7 | 祁海平,杜建豹,蔡元庚.硫酸法钛白废酸及副产硫酸亚铁综合利用[J].山东化工,2021,50(20):256-257,260. |
| QI Haiping, DU Jianbao, CAI Yuangeng.Comprehensive utilization of waste acid and ferrous sulfate from titanium DioxideProduction by sulfuric acid process[J].Shandong Chemical Industry,2021,50(20):256-257,260. | |
| 8 | MAHAZAM N, AZMI N S M.Evaluation of physical and chemical properties of red gypsum from Terengganu,Malaysia[J].International Journal of Engineering Research and Technology,2016,5(1):433-436. |
| 9 | WU Hao, FENG Yali, LI Haoran,et al.Red gypsum utilization and acidic wastewater treatment based on metal self-enrichment process[J].Science of the Total Environment,2019,691:9-15. |
| 10 | 苏念英,黎铉海,卢宇熙,等.机械活化石灰石粉处理钛白废酸制备水泥用石膏[J].有色金属(冶炼部分),2018(11):76-79. |
| SU Nianying, LI Xuanhai, LU Yuxi,et al.Preparation of gypsum for cement from titanium white waste acid treated by mechanical activation lime powder[J].Nonferrous Metals(Extractive Metallurgy),2018(11):76-79. | |
| 11 | 孟华,李琪鹏,石俊杰,等.利用钛白废酸浸出钛石膏铁杂质及技术经济分析[J].化工矿物与加工,2021,50(5):32-37. |
| MENG Hua, LI Qipeng, SHI Junjie,et al.Study on extraction of iron oxide from leaching titanium gypsum using titanium dioxide waste acid and technical & economic performance[J].Industrial Minerals & Processing,2021,50(5):32-37. | |
| 12 | 陈绍鹏.钛白废酸制备α半水石膏及形貌控制[D].郑州:郑州大学,2015. |
| CHEN Shaopeng.Preparation of α-hemihydrate gypsum from titanium dioxide waste acid and its morphology control[D].Zhengzhou:Zhengzhou University,2015. | |
| 13 | 甘永乐,梁艳萍,黄原玲,等.以钛白废酸为原料制备硫酸钙晶须的工艺研究[J].轻工科技,2021,37(8):26-28,118. |
| GAN Yongle, LIANG Yanping, HUANG Yuanling,et al.Study on preparation of calcium sulfate whisker from titanium dioxide waste acid[J].Light Industry Science and Technology,2021, 37(8):26-28,118. | |
| 14 | HAKIM SIDEK M A, MOHD YUNUS R, REMANUL ISLAM M,et al.Physical and mechanical properties of waste red-gypsum based concrete composites[M]//Ismail A,Mohd Daril MA,Öchsner A.Advanced Transdisciplinary Engineering and Technology.Cham:Springer,2022:235-251. |
| 15 | JU Jinrong, FENG Yali, LI Haoran,et al.A novel approach for separation and recovery of titanium,scandium,iron from acidic wastewater and red gypsum utilization[J].Mining,Metallurgy & Exploration,2022,39(3):1297-1312. |
| 16 | 宁延生.无机盐工艺学[M].北京:化学工业出版社,2013. |
| 17 | FUKAMI T, TAHARA S, NAKASONE K,et al.Synthesis,crystal structure,and thermal properties of CaSO4·2H2O single cryst-als[J].International Journal of Chemistry,2015,7(2):12-20. |
| [1] | XIANG Quanjin, QUAN Xuejun, LI Li, WANG Haibo, CHEN Xinhong, LI Ping. Formation rules and emission reduction method of sublimed sulfur in acidolysis exhaust gas of titanium concentrate [J]. Inorganic Chemicals Industry, 2024, 56(7): 96-103. |
| [2] | WANG Luwei, WANG Jie, LI Keke, FENG Chunhua, ZHANG Wenyan, ZHU Jianping. Study on leaching treatment of titanium gypsum with oxalic acid [J]. Inorganic Chemicals Industry, 2024, 56(5): 108-114. |
| [3] | QUAN Yuanxia, QUAN Xuejun, KE Lianghui, LI Li. Study on surface characteristics and dispersion properties of titanium dioxide base particles by sulfate and chloride processes [J]. Inorganic Chemicals Industry, 2024, 56(11): 123-131. |
| [4] | LI Huaquan, QIU Guibao, LÜ Xuewei. Research progress of titanium dioxide preparation technology [J]. Inorganic Chemicals Industry, 2024, 56(10): 20-27. |
| [5] | XIANG Mengqi, MENG Hua, WANG Ye, MENG Xianzhang, BAI Yuhang, WANG Yujunyao, ZHANG Yidan. Study on kinetic of iron leaching from titanium gypsum and its cyclic acid leaching process [J]. Inorganic Chemicals Industry, 2024, 56(1): 114-120. |
| [6] | HU Luoxing,HUANG Qimao,QU Hongyou. Preparation of Fe3O4 from FeCl2 by-product of new process of titanium dioxide by hydrochloric acid [J]. Inorganic Chemicals Industry, 2023, 55(1): 118-123. |
| [7] | ZHU Wanye,TANG Ding,CHI Heting,LIAO Xianghui,ZHUANG Rongchuan,WANG Qiankun,SHEN Qingfeng. Study on impurity removal rule of ferrous sulfate from by-product of titanium dioxide by crystallization purification [J]. Inorganic Chemicals Industry, 2022, 54(7): 105-109. |
| [8] | MA Lei,SHENG Yu,ZHOU Junhong,YANG Yujun,LUO Hui,PAN Chunying,WANG Guo. Study on new process of comprehensive utilization and separation of sulfur and calcium of titanium gypsum [J]. Inorganic Chemicals Industry, 2022, 54(7): 124-128. |
| [9] | TANG Shuyang,GUO Yufeng,ZHENG Fuqiang,CHEN Feng,WANG Shuai,YANG Lingzhi. Present situation and prospects of preparation methods of titanium dioxide [J]. Inorganic Chemicals Industry, 2022, 54(7): 27-34. |
| [10] | WANG Youyou,YUAN Hao,HAN Qingqing,CHEN Shiying. Activation of activator on fly ash?titanium gypsum?calcium carbide slag system and its hydration mechanism [J]. Inorganic Chemicals Industry, 2022, 54(6): 115-119. |
| [11] | Meng Hua,Zhao Jingyi,Nie Chaoyang,Wang Ye. Study on simulation process of reducing titanium dioxide gypsum by sulfur fluidization [J]. Inorganic Chemicals Industry, 2021, 53(9): 61-66. |
| [12] | Chen Wen,Long Xiang,Jin Yaqiu,Mao Duan. Determination of major component in inorganic coated titanium pigment by X-ray fluorescence spectrometry with pressed powder [J]. Inorganic Chemicals Industry, 2021, 53(5): 93-95. |
| [13] | Shu Yirui,Zhang Pan,Wang Wei,Xiang Hengli,Ren Genkuan,Xu Dehua,Zhang Zhiye,Yang Xiushan. Titanium white by-product ferrous sulfate photofenton oxidation degradation of methyl orange in wastewater [J]. Inorganic Chemicals Industry, 2021, 53(3): 68-72. |
| [14] | Wu Yali,Wu Yihan,Zhang Ruimin,Shi Xiaoyu,Dong Xin. Preparation and formation mechanism of hollow barium titanate [J]. Inorganic Chemicals Industry, 2021, 53(2): 51-54. |
| [15] | SUN Chunhui,CHEN Yongsheng,LIU Wei,XU Yan,ZHANG Jingcheng,YU Ruixiang. Study on new preparation process and performance of V2O5 /(TiO2-SiO2) denitrification catalyst [J]. Inorganic Chemicals Industry, 2021, 53(12): 146-149. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||