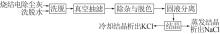
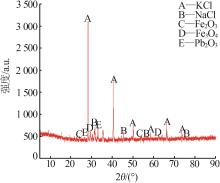
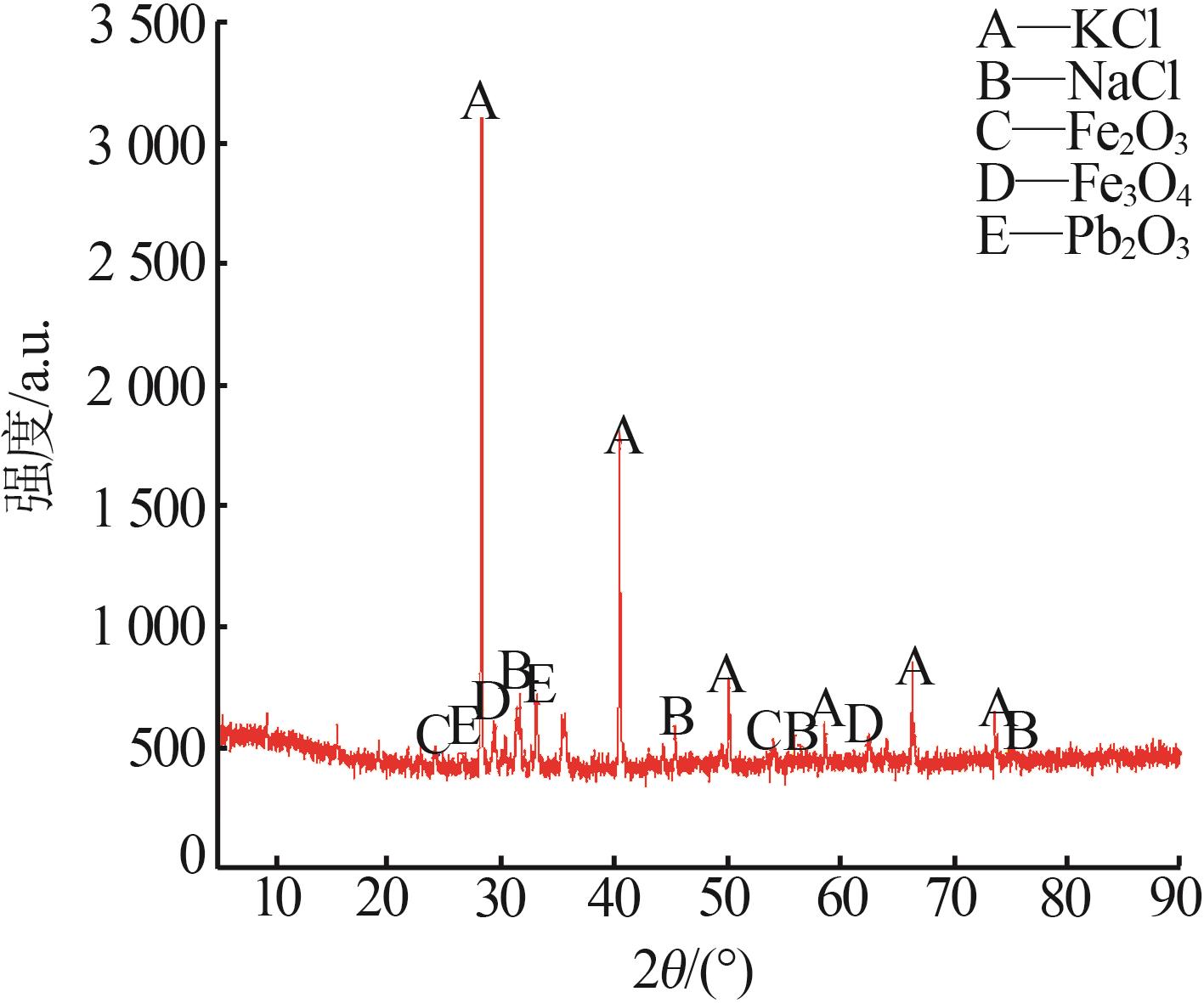
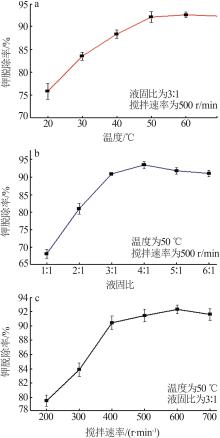
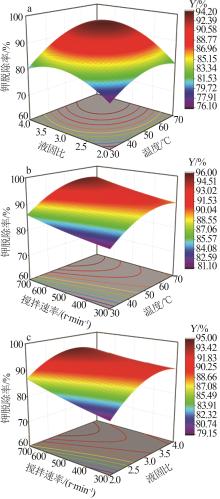
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (6): 102-108.doi: 10.19964/j.issn.1006-4990.2023-0570
• Environment·Health·Safety • Previous Articles Next Articles
Study on leaching extraction of high purity potassium chloride from sintering machine head ash
CHEN Junhui1( ), LIU Xiang2, HU Qingxi1(
), LIU Xiang2, HU Qingxi1( ), TIAN Bangxin2, CHEN Jiale2
), TIAN Bangxin2, CHEN Jiale2
- 1.State Key Laboratory of Pulp and Paper Engineering,South China University of Technology,Guangzhou 510641,China
2.Guangzhou Maiyuan Technology Co. ,Ltd. ,Guangzhou 510700,China
-
Received:2023-08-29Online:2024-06-10Published:2024-06-20 -
Contact:HU Qingxi E-mail:2727801361@qq.com;qxhu@scut.edu.cn
CLC Number:
Cite this article
CHEN Junhui, LIU Xiang, HU Qingxi, TIAN Bangxin, CHEN Jiale. Study on leaching extraction of high purity potassium chloride from sintering machine head ash[J]. Inorganic Chemicals Industry, 2024, 56(6): 102-108.
share this article
Table 4
Response surface design experiment and results"
| 序号 | A | B | C | Y/% |
|---|---|---|---|---|
| 1 | 30 | 2∶1 | 500 | 75.62 |
| 2 | 70 | 2∶1 | 500 | 82.26 |
| 3 | 30 | 4∶1 | 500 | 79.26 |
| 4 | 70 | 4∶1 | 500 | 93.86 |
| 5 | 30 | 3∶1 | 300 | 82.77 |
| 6 | 70 | 3∶1 | 300 | 89.60 |
| 7 | 30 | 3∶1 | 700 | 86.13 |
| 8 | 70 | 3∶1 | 700 | 93.87 |
| 9 | 50 | 2∶1 | 300 | 78.17 |
| 10 | 50 | 4∶1 | 300 | 89.21 |
| 11 | 50 | 2∶1 | 700 | 87.43 |
| 12 | 50 | 4∶1 | 700 | 93.74 |
| 13 | 50 | 3∶1 | 500 | 90.76 |
| 14 | 50 | 3∶1 | 500 | 92.53 |
| 15 | 50 | 3∶1 | 500 | 91.75 |
| 16 | 50 | 3∶1 | 500 | 91.56 |
| 17 | 50 | 3∶1 | 500 | 90.52 |
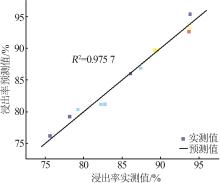
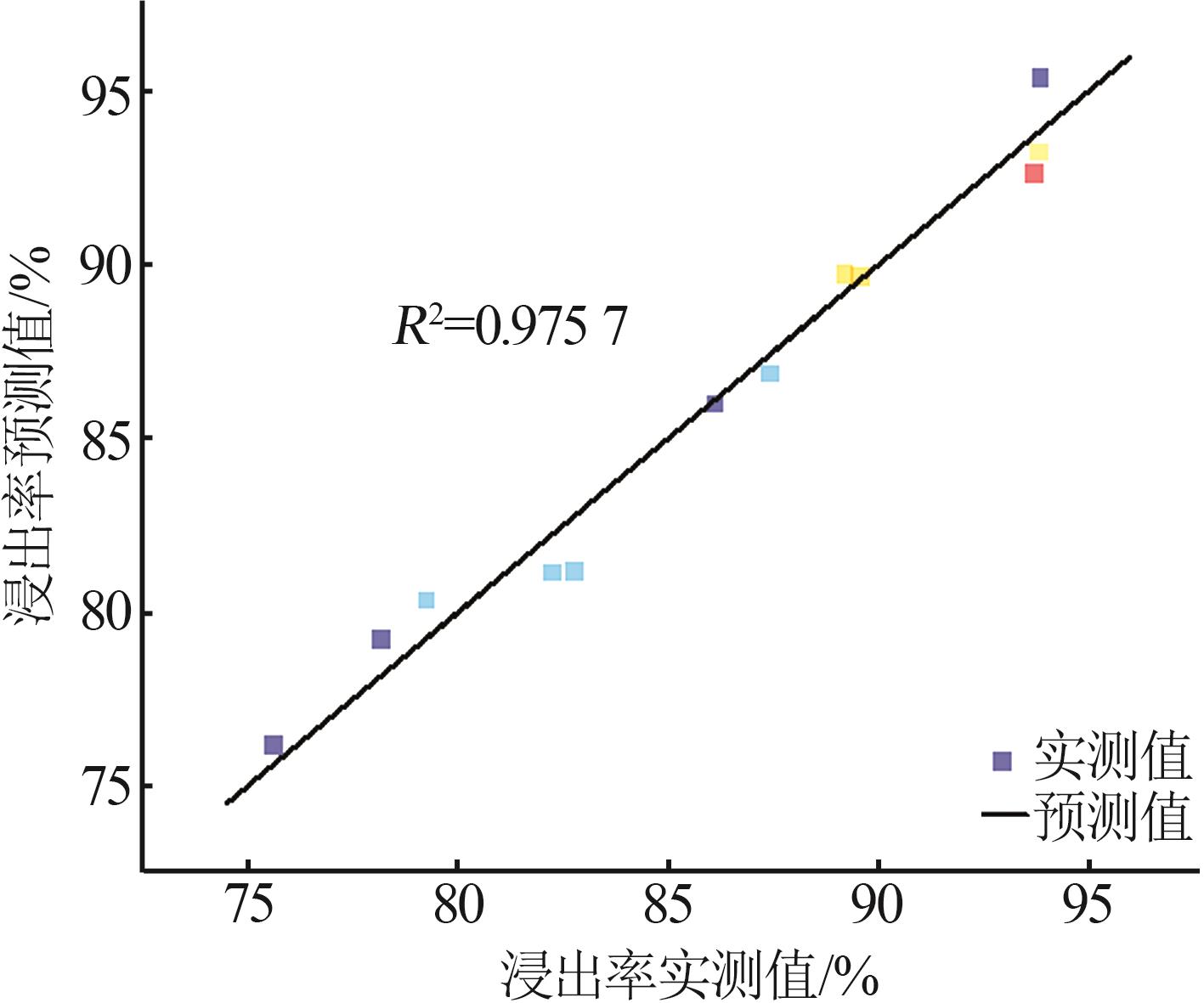
Table 5
Results of variance analysis of regression model Y"
| 项目 | 均方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 模型 | 541.08 | 9 | 60.12 | 31.25 | < 0.000 1 | 显著 |
| A | 160.29 | 1 | 160.29 | 83.32 | < 0.000 1 | |
| B | 132.76 | 1 | 132.76 | 69.01 | < 0.000 1 | |
| C | 57.35 | 1 | 57.35 | 29.81 | 0.000 9 | |
| AB | 15.84 | 1 | 15.84 | 8.23 | 0.024 | |
| AC | 0.207 | 1 | 0.207 | 0.107 6 | 0.752 5 | |
| BC | 5.59 | 1 | 5.59 | 2.91 | 0.132 | |
| A2 | 62.72 | 1 | 62.72 | 32.6 | 0.000 7 | |
| B2 | 97.6 | 1 | 97.6 | 50.73 | 0.000 2 | |
| C2 | 1.17 | 1 | 1.17 | 0.610 1 | 0.460 3 | |
| 残差 | 13.47 | 7 | 1.92 | |||
| 失拟差 | 10.86 | 3 | 3.62 | 5.56 | 0.065 5 | 不显著 |
| 纯误差 | 2.61 | 4 | 0.651 5 | |||
| 总和 | 554.55 | 16 |
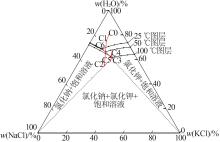
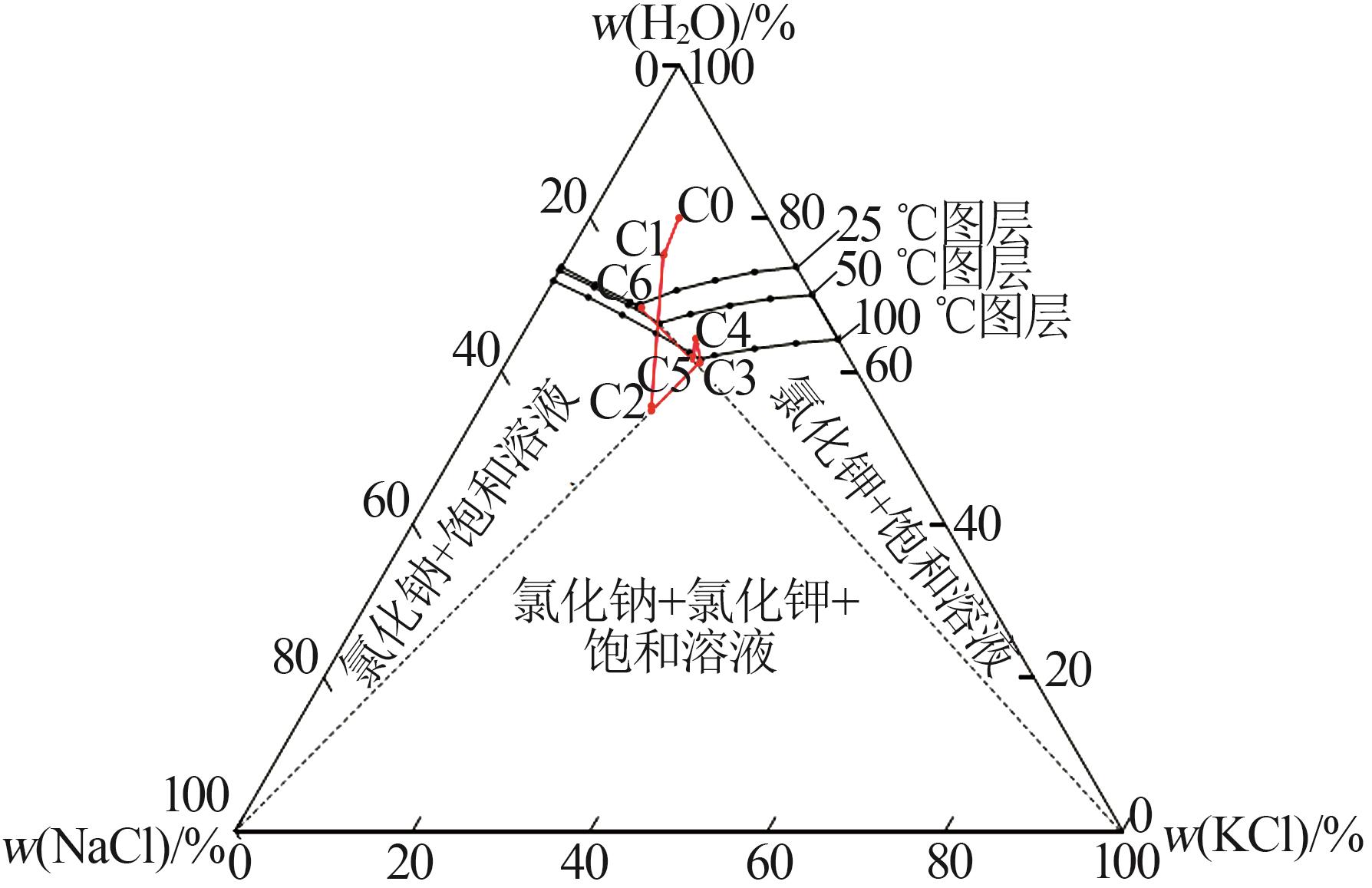
Table 7
Discharge concentration at eachdesigned phase plot site"
操作点 温度/℃ | 物料 | 质量分数/% | 相图 点位 | |
|---|---|---|---|---|
| NaCl | KCl | |||
| 0 | 原水 | 10.26 | 10.71 | C0 |
| 25 | 回流母液 | 18.76 | 11.45 | C6 |
| 12 | 三效混合进料 | 14.19 | 10.69 | C1 |
| 110 | 三效蒸发终点(固混) | 23.99 | 18.07 | C2 |
| 110 | 三效蒸发终点(液相) | 15.88 | 20.00 | C3 |
| 45~50 | 闪发起点(兑卤后) | 15.22 | 18.87 | C4 |
| 45~50 | 闪发结晶终点(固混) | 16.47 | 20.42 | C5 |
| 45~50 | 闪发结晶终点(液相) | 17.60 | 14.95 | C5 |
| 25 | 冷却结晶终点(液相) | 18.76 | 11.45 | C6 |
| 1 | 侯殿保,贺茂勇,陈育刚,等.资源优化配置与循环经济在钾资源开发利用中的应用[J].化工进展,2023,42(6):3197-3208. |
| HOU Dianbao, HE Maoyong, CHEN Yugang,et al.Application analysis of resource allocation optimization and circular economy in development and utilization of potassium resources[J].Chemical Industry and Engineering Progress,2023,42(6):3197-3208. | |
| 2 | 张硕.中国钾盐供需形势分析[D].北京:中国地质大学(北京),2021:35-80. |
| ZHANG Shuo.Analysis of potash resource supply and demand situation in china[D].Beijing:China University of Geosciences,2021:35-80. | |
| 3 | WAKEEL A, ISHFAQ M.Potash Use and Dynamics in Agricultu-re[M].Singapore:Springer Singapore,2022. |
| 4 | 罗婷,张永庆,郑明贵,等.中国钾盐资源安全评估与预警研究[J].地球科学进展,2022,37(6):575-587. |
| LUO Ting, ZHANG Yongqing, ZHENG Minggui,et al.Security assessment and early warning of potash resources in China[J].Advances in Earth Science,2022,37(6):575-587. | |
| 5 | 张梅,付志刚,吴滨,等.钢铁冶金烧结机头电除尘灰中氯化钾的回收[J].过程工程学报,2014,14(6):979-983. |
| ZHANG Mei, FU Zhigang, WU Bin,et al.Recovery of potassium chloride from sintering EAF dust[J].The Chinese Journal of Process Engineering,2014,14(6):979-983. | |
| 6 | 刘宪,蒋新民,杨余,等.烧结机头电除尘灰中钾的脱除及利用其制备硫酸钾[J].金属材料与冶金工程,2011,39(3):40-45, 57. |
| LIU Xian, JIANG Xinmin, YANG Yu,et al.Removal of potassium and preparation of potassium sulfate from sintering EAF dust[J].Metal Materials and Metallurgy Engineering,2011,39(3):40-45,57. | |
| 7 | YU Yaowei, WANG Ziming, WEI Han,et al.Separation and recovery of potassium chloride from sinter dust of a steel plant[J].Ironmaking&Steelmaking,2019,46(2):193-198. |
| 8 | 李亚娇,赵艺伟,鞠恺,等.基于响应面法的粉煤灰氨含量测定过程浸提条件优化研究[J].无机盐工业,2022,54(4):145- 151. |
| LI Yajiao, ZHAO Yiwei, JU Kai,et al.Study on optimization of extraction conditions in process of determination of ammonia content in fly ash based on response surface method[J].Inorganic Chemicals Industry,2022,54(4):145-151. | |
| 9 | 黄继明,刘润清,吴思展,等.响应面法优化氯化铵焙烧浸出低品位菱锰矿工艺[J].无机盐工业,2019,51(3):34-37. |
| HUANG Jiming, LIU Runqing, WU Sizhan,et al.Optimization of extraction process of chlorination roasting-water leaching process for low-grade rhodochrosite by response surface methodology[J].Inorganic Chemicals Industry,2019,51(3):34-37. | |
| 10 | 胡渝杰,叶国华,左琪,等.响应曲面法优化承钢含钒钢渣浸出负组元钙的试验研究[J].有色金属工程,2021,11(5):52- 59. |
| HU Yujie, YE Guohua, ZUO Qi,et al.Study on optimization of leaching of negative component calcium from vanadium-bearing slag of Chenggang by response surface methodology[J].Nonferrous Metals Engineering,2021,11(5):52-59. | |
| 11 | 方伟成,程星星,孙常荣.响应曲面法优化污泥/粉煤灰复合陶粒滤料的制备[J].无机盐工业,2022,54(9):119-125,142. |
| FANG Weicheng, CHENG Xingxing, SUN Changrong.Optimization of preparation of sludge/fly ash composite ceramsite filler materials by response surface methodology[J].Inorganic Chemicals Industry,2022,54(9):119-125,142. | |
| 12 | 叶铁林.化工结晶过程原理及应用[M].3版.北京:北京工业大学出版社,2020:3-22. |
| 13 | 邓天龙,周桓,陈侠.水盐体系相图及应用[M].2版.北京:化学工业出版社,2020:54-100. |
| 14 | JENA S K, MOHANTY B, PADHY G,et al.Potassium recovery from muscovite using NaCl-roasting followed by H2SO4-leachi-ng[J].Journal of Central South University,2022,29(6):1881-1894. |
| [1] | TANG Dongwu, YE Changwen, DENG Jie, AO Fang. Study on leaching rate of calcium and magnesium from phosphorus tailings based on thermodynamic analysis and response surface method [J]. Inorganic Chemicals Industry, 2024, 56(9): 98-106. |
| [2] | GUO Kaihua, FAN Yuxin, YANG Jing, ZHAO Wenli, JIA Yuanyuan, WANG Yanfei. Analysis of effect of carnallite raw ore grade on its cold decomposition and crystallization of potassium chloride [J]. Inorganic Chemicals Industry, 2024, 56(8): 9-18. |
| [3] | CHENG Chunchun, LI Yulong, ZHANG Zhiqiang, LIU Xuejing. Study on dissolution crystallization for extraction of potassium and separation of magnesium and lithium from salt lake brine [J]. Inorganic Chemicals Industry, 2024, 56(6): 34-39. |
| [4] | LIU Xiaowen, LI Jun, ZHOU Zhaoan, MAO Anzhang, ZHOU Aiqing. Study on response surface methodology optimization of PAC for deep purification of fluorine ion in high concentration sodium sulfate solution [J]. Inorganic Chemicals Industry, 2024, 56(6): 67-72. |
| [5] | LI Chunli, ZHANG Huanhuan, CHENG Zhuo, TANG Xiuhua, ZHANG Fengzhen, YE Yuling. Anti-solvent crystallization process of NH4VO3 in NaVO3-NH4Cl-H2O solution system [J]. Inorganic Chemicals Industry, 2024, 56(5): 39-44. |
| [6] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [7] | DING Xiaojiang, WU Yanni, LI Boyun, HUANG Youliang. Research on preparation of carnallite concentrate by pretreatment combined with reverse flotation [J]. Inorganic Chemicals Industry, 2024, 56(12): 113-119. |
| [8] | YANG Bo, MA Zhen, ZENG Ying, YAN Xiongzhong, LI Qi, HOU Yuansheng, YU Xudong. Study on solid-liquid phase equilibria in ternary system of Li+(K+),Rb+//Cl--H2O at 288.2 K [J]. Inorganic Chemicals Industry, 2024, 56(11): 116-122. |
| [9] | HU Mingliang, ZHOU Wei, LI Bin, LAI Xiaoling. Research progress of synergistic effect catalytic reforming of methane and carbon dioxide [J]. Inorganic Chemicals Industry, 2024, 56(1): 23-32. |
| [10] | ZHANG Conghua, YAN Wenbin, XIAO Jiajun, ZHAO Ke, PENG Shangquan, WEI Yuhong. Reductive leaching technology of manganese anode slag using tartaric acid as reducing agent optimized by RSM [J]. Inorganic Chemicals Industry, 2023, 55(9): 106-113. |
| [11] | WANG Yingnan, SHENG Linlin, HUANG Juan, HUANG Zhanbin. Study on adsorption performance of lead from water by coal-fired slag [J]. Inorganic Chemicals Industry, 2023, 55(8): 109-115. |
| [12] | CUI Gengyin, XIE Lang, LU Yuexian, KONG Dewen, WANG Lingling. Optimization of mechanical properties of basalt fiber reinforced phosphogypsum-based composites based on RSM [J]. Inorganic Chemicals Industry, 2023, 55(8): 116-123. |
| [13] | FENG Zhun. Improvement of high temperature stability of high nickel single crystal cathode materials by B/Al/Zr synergistic strategy [J]. Inorganic Chemicals Industry, 2023, 55(8): 59-64. |
| [14] | LI Yan, MA Zhen, SONG Xingfu. New development advance and industrial development proposals of chloride-type salt lake potassium resources in Qinghai [J]. Inorganic Chemicals Industry, 2023, 55(8): 84-90. |
| [15] | MI Xiaotong, LI Xiaoguo, CHANG Yang, ZHANG Yongkun, LI Yongheng, CAO Hui, HOU Zhanggui. Collaborative construction of nanoscale ZSM-5 aggregates by crystal seeds and templates [J]. Inorganic Chemicals Industry, 2023, 55(6): 130-135. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||