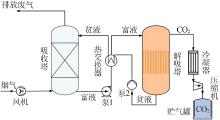
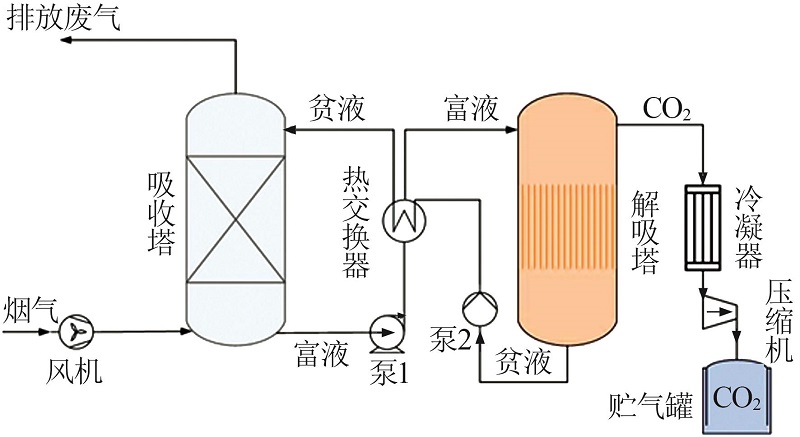
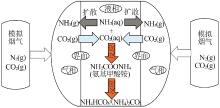
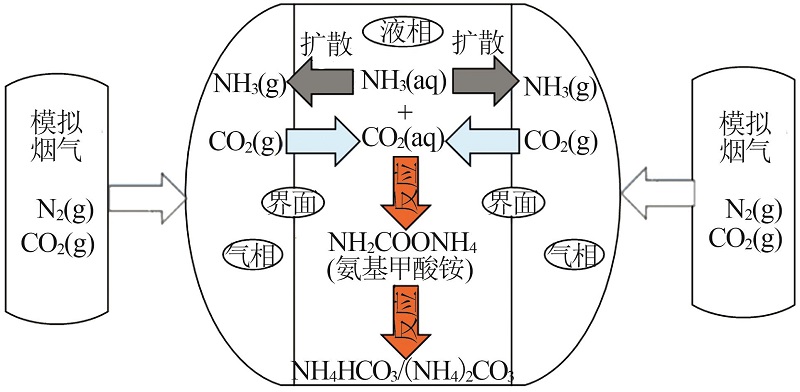
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (10): 79-86.doi: 10.19964/j.issn.1006-4990.2021-0684
• Reviews and Special Topics • Previous Articles Next Articles
Research progress on effect of inorganic additives on regeneration process of carbon capture by ammonia
QIAO Kun( ),LÜ Zening,YANG Lijun(
),LÜ Zening,YANG Lijun( ),DU Xiaoze
),DU Xiaoze
- Key Laboratory of Power Station Energy Transfer Conversion and System of Ministry of Education,School of Energy Power and Mechanical Engineering,North China Electric Power University,Beijing 102206,China
-
Received:2021-11-12Online:2022-10-10Published:2022-11-03 -
Contact:YANG Lijun E-mail:18810731655@163.com;yanglj@ncepu.edu.cn
CLC Number:
Cite this article
QIAO Kun,LÜ Zening,YANG Lijun,DU Xiaoze. Research progress on effect of inorganic additives on regeneration process of carbon capture by ammonia[J]. Inorganic Chemicals Industry, 2022, 54(10): 79-86.
share this article
Table 1
Partial summary of additive effects in the absorption process"
| 添加剂名称 | 类型 | 实验条件 | 性能评价 |
|---|---|---|---|
| 乙醇[ | 有机 | 反应温度为20 ℃,氨质量分数为5% | 反应4 h时,CO2脱除效率能达到95%,比空白组高10%,抑制氨逃逸效果研究较少 |
| 氨甲基丙醇、氨甲基丙二醇、烯丙基乙基丙二醇、氨基丁三醇[ | 有机 | 反应温度为25 ℃,氨质量分数为10%,压力为0.1 MPa;反应温度为40 ℃,氨质量分数为 10%,压力为0.1 MPa | 其中氨甲基丙二醇CO2脱除效率最高,最高能达到71%,其余添加剂最高达到60%;抑制氨逃逸效果由弱到强分别是氨甲基丙二醇、烯丙基乙基丙二醇、氨甲基丙醇、氨基丁三醇,其中氨基丁三醇抑制率接近52% |
| 聚乙二醇二甲醚[ | 有机 | 反应温度为20 ℃,氨质量分数为1.2% | 抑制氨逃逸效果达到24.8% |
| 乙二醇、甘油、甘氨酸[ | 有机 | 反应温度为40 ℃,氨质量分数为9%,添加剂质量分数为1% | 在CO2吸收过程中抑制氨效果最好的是甘油,达到63.9%,接下来是乙二醇、甘氨酸 |
| ZnCl2、Zn(NO3)2、ZnSO4[ | 无机 | 压力为0.1 MPa,反应温度为0 ℃,氨质量分数为0.77% | 平均CO2脱除效率为87.9%~89.4%,并且锌盐对其促进脱除效率影响较为轻微 |
| Cu(OH)2[ | 无机 | 常压,氨质量分数为9%,反应温度为30 ℃,CO2体积分数为20% | CO2的脱除率达到95%以上,质量浓度为0.2 g/L的 Cu(OH)2加入后抑制氨效果最好,约为40%,其效果优于0.1 g/L和0.5 g/L的Cu(OH)2 |
| CoCl2[ | 无机 | 反应温度为15 ℃,氨质量分数为8%,CO2体积分数为15% | 实验条件下Co离子的最佳添加浓度为0.05 mol/L左右,氨逃逸抑制效率为40.45%,且改变实验温度时,随着温度的增加,抑制效果提高 |
| [1] | LIU Fujian, HUANG Kuan, JIANG Lilong.Promoted adsorption of CO2 on amine-impregnated adsorbents by functionalized ionic liquids[J].AIChE Journal, 2018, 64(10): 3671-3680. |
| [2] | 纪龙, 曾鸣.燃煤电厂CO2捕集与利用技术综述[J].煤炭工程, 2014, 46(3): 90-92. |
| JI Long, ZENG Ming.Review on capture and utilization technology of carbon dioxide in coal-fired power plant[J].Coal Engineering, 2014, 46(3): 90-92. | |
| [3] | STERN N.Stern review:The economics of climate change[J].Nature, 2007, 378(6556): 433. |
| [4] | 张九天, 张璐.面向碳中和目标的碳捕集、利用与封存发展初步探讨[J].热力发电, 2021, 50(1): 1-6. |
| ZHANG Jiutian, ZHANG Lu.Preliminary discussion on development of carbon capture,utilization and storage for carbon neutralization[J].Thermal Power Generation, 2021, 50(1): 1-6. | |
| [5] | 张力为, 甘满光, 王燕, 等.二氧化碳捕集利用-可再生能源发电调峰耦合技术[J].热力发电, 2021, 50(1): 24-32. |
| ZHANG Liwei, GAN Manguang, WANG Yan, et al.Coupled technology of carbon dioxide capture and utilization and renewable power peak shaving[J].Thermal Power Generation, 2021, 50(1): 24-32. | |
| [6] | LEUNG D Y C, CARAMANNA G, MAROTO-VALER M M.An overview of current status of carbon dioxide capture and storage technologies[J].Renewable and Sustainable Energy Reviews, 2014, 39: 426-443. |
| [7] | HASZELDINE R S.Carbon capture and storage:How green can black Be?[J].Science, 2009, 325(5948): 1647-1652. |
| [8] | OECD.Energy technology perspectives 2020[M].Paris: Organisation for Economic Co-operation and Development, 2020. |
| [9] | 魏宁, 姜大霖, 刘胜男, 等. 国家能源集团燃煤电厂CCUS改造的成本竞争力分析[J].中国电机工程学报, 2020, 40(4): 1258-1265, 1416. |
| WEI Ning, JIANG Dalin, LIU Shengnan, et al.Cost competitiveness analysis of retrofitting CCUS to coal-fired power plants[J].Proceedings of the CSEE, 2020, 40(4): 1258-1265, 1416. | |
| [10] | LIANG Zhiwu, FU Kaiyun, IDEM R, et al.Review on current advances,future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents[J].Chinese Journal of Chemical Engineering, 2016, 24(2): 278-288. |
| [11] | WANG Peng, GUO Yafei, ZHAO Chuanwen, et al. Biomass derived wood ash with amine modification for post-combustion CO2 capture[J].Applied Energy, 2017, 201: 34-44. |
| [12] | JACOBSON M Z.Review of solutions to global warming,air pollution,and energy security[J].Energy & Environmental Science, 2009, 2(2): 148-173. |
| [13] | LI Jianrong, MA Yuguang, MCCARTHY M C, et al.Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks[J].Coordination Chemistry Reviews, 2011, 255(15/16): 1791-1823. |
| [14] | D′ALESSANDRO D M, SMIT B, LONG J R.Carbon dioxide capture:Prospects for new materials[J].Angewandte Chemie(International Ed.in English), 2010, 49(35): 6058-6082. |
| [15] | XIAO Min, LIU Helei, IDEM R, et al.A study of structure-activity relationships of commercial tertiary amines for post-combustion CO2 capture[J].Applied Energy, 2016, 184: 219-229. |
| [16] | VALENTI G, BONALUMI D, FOSBØL P, et al.Alternative layouts for the carbon capture with the chilled ammonia process[J].Energy Procedia, 2013, 37: 2076-2083. |
| [17] | BEN-MANSOUR R, HABIB M A, BAMIDELE O E, et al.Carbon capture by physical adsorption:Materials,experimental investigations and numerical modeling and simulations-A review[J].Applied Energy, 2016, 161: 225-255. |
| [18] | LIANG Zhiwu, RONGWONG W, LIU Helei, et al.Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents[J].International Journal of Greenhouse Gas Control, 2015, 40: 26-54. |
| [19] | YANG Shitu, YU Chenxi, YU Lili, et al.Bridging dealumination and desilication for the synthesis of hierarchical MFI zeolites[J].Angewandte Chemie:International Ed.in English, 2017, 56(41): 12553-12556. |
| [20] | CHAKRAVARTI S, AMITABH G, BALAZS H.Advanced technology for the capture of carbon dioxide from flue gases[C]//First National Conference on Carbon Sequestration.Washington D C, 2001: 1-11. |
| [21] | TOBIESEN F A, SVENDSEN H F, JULIUSSEN O.Experimental validation of a rigorous absorber model for CO2 postcombustion capture[J].AIChE Journal, 2007, 53(4): 846-865. |
| [22] | SHAKERIAN F, KIM K H, SZULEJKO J E, et al.A comparative review between amines and ammonia as sorptive media for post-combustion CO2 capture[J].Applied Energy, 2015, 148: 10-22. |
| [23] | CHENG C H, LI Kangkang, YU Hai, et al.Amine-based post-combustion CO2 capture mediated by metal ions:Advancement of CO2 desorption using copper ions[J].Applied Energy, 2018, 211: 1030-1038. |
| [24] | BARZAGLI F, GIORGI C, MANI F, et al.Reversible carbon dioxide capture by aqueous and non-aqueous amine-based absorbents:A comparative analysis carried out by 13C NMR spectroscopy[J].Applied Energy, 2018, 220: 208-219. |
| [25] | VEVELSTAD S J, EIDE-HAUGMO I, SILVA E F DA, et al.Degradation of MEA;a theoretical study[J].Energy Procedia, 2011, 4: 1608-1615. |
| [26] | RUBIN E S, MANTRIPRAGADA H, MARKS A, et al.The outlook for improved carbon capture technology[J].Progress in Energy and Combustion Science, 2012, 38(5): 630-671. |
| [27] | YU Hai.Recent developments in aqueous ammonia-based post-combustion CO2 capture technologies[J].Chinese Journal of Che- mical Engineering, 2018, 26(11): 2255-2265. |
| [28] | DARDE V, VAN WELL W J M, FOSBOEL P L, et al.Experimental measurement and modeling of the rate of absorption of carbon dioxide by aqueous ammonia[J].International Journal of Greenhouse Gas Control, 2011, 5(5): 1149-1162. |
| [29] | ZHANG Minkai, GUO Yincheng.Process simulations of large-scale CO2 capture in coal-fired power plants using aqueous ammonia solution[J].International Journal of Greenhouse Gas Control, 2013, 16: 61-71. |
| [30] | YU Hai, QI Guojie, WANG Shujuan, et al.Results from trialling aqueous ammonia-based post-combustion capture in a pilot plant at Munmorah power station:Gas purity and solid precipitation in the stripper[J].International Journal of Greenhouse Gas Control, 2012, 10: 15-25. |
| [31] | YU Hai, XIANG Qunyang, FANG Mengxiang, et al.Promoted CO2 absorption in aqueous ammonia[J].Greenhouse Gases:Science and Technology, 2012, 2(3): 200-208. |
| [32] | YEH J T, RESNIK K P, RYGLE K, et al.Semi-batch absorption and regeneration studies for CO2 capture by aqueous ammonia[J].Fuel Processing Technology, 2005, 86(14/15): 1533-1546. |
| [33] | 马双忱, 陈公达, 马宵颖, 等. 氨法碳捕集过程中氨逃逸控制[J].化工学报, 2014, 65(10): 4086-4093. |
| MA Shuangchen, CHEN Gongda, MA Xiaoying, et al.Ammonia escape control in carbon dioxide capture using ammonia method[J].CIESC Journal, 2014, 65(10): 4086-4093. | |
| [34] | PELLEGRINI G, STRUBE R, MANFRIDA G.Comparative study of chemical absorbents in postcombustion CO2 capture[J].Energy, 2010, 35(2): 851-857. |
| [35] | YOU J K, PARK H, YANG S H, et al.Influence of additives including amine and hydroxyl groups on aqueous ammonia absorbent for CO2 capture[J].The Journal of Physical Chemistry.B, 2008, 112(14): 4323-4328. |
| [36] | 马双忱, 孙云雪, 崔基伟, 等. 聚乙二醇二甲醚抑制脱碳吸收剂中氨逃逸的实验及原理分析[J].化工学报, 2011, 62(5): 1408-1413. |
| MA Shuangchen, SUN Yunxue, CUI Jiwei, et al.Experiment and analysis of ammonia escape from decarburization absorbent inhibited by NHD[J].CIESC Journal, 2011, 62(5): 1408-1413. | |
| [37] | SEO J B, JEON S B, KIM J Y, et al.Vaporization reduction characteristics of aqueous ammonia solutions by the addition of ethylene glycol,glycerol and glycine to the CO2 absorption process[J].Journal of Environmental Sciences, 2012, 24(3): 494-498. |
| [38] | MANI F, PERUZZINI M, BARZAGLI F.The role of zinc(Ⅱ) in the absorption-desorption of CO2 by aqueous NH3,a potentially cost-effective method for CO2 capture and recycling[J].ChemSusChem, 2008, 1(3): 228-235. |
| [39] | KIM Y, LIM S R, PARK J M.The effects of Cu(Ⅱ) ion as an additive on NH3 loss and CO2 absorption in ammonia-based CO2 capture processes[J].Chemical Engineering Journal, 2012, 211-212: 327-335. |
| [40] | 郑志胜, 钦淑均, 沈小耀, 等. 低碳化度氨水吸收二氧化碳速率的研究[J].华东化工学院学报, 1984, 10(2): 137-146. |
| ZHENG Zhisheng, QIN Shujun, SHEN Xiaoyao, et al.A study on absorption rates of CO2 into low degree carbonated ammonia-water solutions[J].Journal of East China Institute of Chemical Technology, 1984, 10(2): 137-146. | |
| [41] | ZHANG Y, LI Zhenzhong, LI Xin, et al.Preliminary study to capture CO2 in flue gas by spraying aqueous ammonia to produce NH4HCO3 [C]//Second Annual Conference on Carbon Sequestration.Alexandria, 2003. |
| [42] | BAI H, YEH A C.Removal of CO2 greenhouse gas by ammonia scrubbing[J].Industrial & Engineering Chemistry Research, 1997, 36(6): 2490-2493. |
| [43] | 马双忱, 孙云雪, 赵毅, 等. 氨水捕集模拟烟气中二氧化碳的实验与理论研究[J].化学学报, 2011, 69(12): 1469-1474. |
| MA Shuangchen, SUN Yunxue, ZHAO Yi, et al.Experimental and mechanism research on CO2 capture from simulating flue gas using ammonia solution[J].Acta Chimica Sinica, 2011, 69(12): 1469-1474. | |
| [44] | 王甫, 赵军, 邓帅, 等. 氨法碳捕集中氨逃逸抑制机制研究进展[J].化工进展, 2017, 36(12): 4641-4650. |
| WANG Fu, ZHAO Jun, DENG Shuai, et al.Review on development and mechanism of reducing ammonia escape from carbon dioxide capture process using ammonia method[J].Chemical Industry and Engineering Progress, 2017, 36(12): 4641-4650. | |
| [45] | MA Shuangchen, SONG Huihui, ZANG Bin, et al.Experimental study of Co(Ⅱ) additive on ammonia escape in carbon capture using renewable ammonia[J].Chemical Engineering Journal, 2013, 234: 430-436. |
| [46] | LI Kangkang, YU Hai, TADE M, et al.Theoretical and experimental study of NH3 suppression by addition of Me(Ⅱ) ions (Ni,Cu and Zn) in an ammonia-based CO2 capture process[J].International Journal of Greenhouse Gas Control, 2014, 24: 54-63 |
| [47] | 陈公达.基于氨法碳捕集的工艺环节优化与吸收剂改性实验研究[D].北京:华北电力大学(北京), 2017. |
| CHEN Gongda.Experimental research on process optimization and absorbent modification in ammonia-based carbon capture[D].Beijing:North China Electric Power University, 2017. | |
| [48] | 刘溪.添加剂对氨法吸收解吸CO2性能的影响研究[D].南京:东南大学, 2014. |
| LIU Xi.Experimental research on effects of additives to the ammo-nia-based CO2 capture process[D].Nanjing:SouthEast University,2014. | |
| [49] | RESNIK K P, GARBER W, HREDA D C, et al.A parametric study for regenerative ammonia-based scrubbing for the capture of CO2 [C]//Proceedings of 23rd Annual International Pittsburgh Coal Conference.Pittsburgh, 2006. |
| [50] | BHATTI U H, SHAH A K, KIM J N, et al.Effects of transition metal oxide catalysts on MEA solvent regeneration for the post-combustion carbon capture process[J].ACS Sustainable Chemistry & Engineering, 2017, 5(7): 5862-5868. |
| [51] | SHI Huancong, NAAMI A, IDEM R, et al.Catalytic and non catalytic solvent regeneration during absorption-based CO2 capture with single and blended reactive amine solvents[J].International Journal of Greenhouse Gas Control, 2014, 26: 39-50. |
| [52] | OSEI P A, AKACHUKU A, DECARDI-NELSON B, et al.Mass transfer studies on catalyst-aided CO2 desorption from CO2-loaded amine solution in a post-combustion CO2 capture plant[J].Chemi-cal Engineering Science, 2017,170: 508-517. |
| [53] |
SALEH BAIRQ Z ALI, GAO Hongxia, HUANG Yufei, et al.Enhancing CO2 desorption performance in rich MEA solution by addition of SO4 2-/ZrO2/SiO2 bifunctional catalyst[J].Applied Energy, 2019,252.Doi:10.1016/j.apenergy.2019.113440 .
doi: 10.1016/j.apenergy.2019.113440 |
| [54] | ZHANG Xiaowen, ZHU Zhiqing, SUN Xiaoyu, et al.Reducing energy penalty of CO2 capture using Fe promoted SO4 2-/ZrO2/MCM-41 catalyst[J].Environmental Science & Technology, 2019, 53(10): 6094-6102. |
| [55] | MURNANDARI A, KANG Jimin, YOUN M H, et al.Effect of process parameters on the CaCO3 production in the single process for carbon capture and mineralization[J].Korean Journal of Che- mical Engineering, 2017, 34(3): 935-941. |
| [56] | JI Long, YU Hai, LI Kangkang, et al.Integrated absorption-mineralisation for low-energy CO2 capture and sequestration[J].Applied Energy, 2018, 225: 356-366. |
| [57] | YU Bing, LI Kangkang, JI Long, et al.Coupling a sterically hindered amine-based absorption and coal fly ash triggered amine regeneration:A high energy-saving process for CO2 absorption and sequestration[J].International Journal of Greenhouse Gas Control, 2019, 87: 58-65. |
| [58] | 吕春捷, 李孟盈, 徐立华, 等. 复配醇胺水溶液捕集CO2过程中的解吸研究进展[J].应用化工, 2021, 50(1): 238-243, 249. |
| Chunjie LÜ, LI Mengying, XU Lihua, et al.Research progress in desorption of CO2 capture by blended amine aqueous solution[J].Applied Chemical Industry, 2021, 50(1): 238-243, 249. | |
| [59] | WANG Tao, YU Wei, LIU Fei, et al.Enhanced CO2 absorption and desorption by monoethanolamine(MEA)-based nanoparticle suspensions[J].Industrial & Engineering Chemistry Research, 2016, 55(28): 7830-7838. |
| [60] | ZHANG Qi, CHENG Congcong, WU Tao, et al.The effect of Fe3O4 nanoparticles on the mass transfer of CO2 absorption into aqueous ammonia solutions[J].Chemical Engineering and Proce-Intensification ssing-Process, 2020,154.Doi:10.1016/j.cep.2020.108002. |
| [61] | 柴彤, 赵瑞红, 栗明宏, 等. 氨基改性有序介孔氧化铝吸附二氧化碳性能研究[J].无机盐工业, 2016, 48(12): 14-18. |
| CHAI Tong, ZHAO Ruihong, LI Minghong, et al.Adsorption of CO2 with amino-modified ordered mesoporous alumina[J].Inorganic Chemicals Industry, 2016, 48(12): 14-18. |
| [1] | YAN Xin, LIU Hailu, LIU Baolin, LIU Yi, LIU Yanyang. Research on key technologies and mechanisms of green nano calcium carbonate production [J]. Inorganic Chemicals Industry, 2025, 57(1): 71-76. |
| [2] | ZHAN Sijin, LIU Shike, LIU Fei, YAO Mengqin, CAO Jianxin. Study on preparation and catalytic performance of ZnO-CeO2 [J]. Inorganic Chemicals Industry, 2024, 56(3): 137-143. |
| [3] | REN Qixia, YANG Kun, LIU Fei, YAO Mengqin, CAO Jianxin. Effect of promoter on physicochemical properties and catalytic performance of ZnO/ZrO2 [J]. Inorganic Chemicals Industry, 2024, 56(3): 144-154. |
| [4] | YANG Kun, REN Qixia, DONG Yonggang, LIU Fei, YAO Mengqin, CAO Jianxin. Effect of calcination temperature on catalytic performance of ZnGaZrO x /SAPO-34 [J]. Inorganic Chemicals Industry, 2024, 56(2): 136-145. |
| [5] | LI Yang, LOU Feijian, SUI Xin, LI Keyan, LIU Fei, GUO Xinwen. Preparation of amine-functionalized fumed SiO2 materials and their performance for CO2 adsorption [J]. Inorganic Chemicals Industry, 2024, 56(2): 38-43. |
| [6] | HOU Zhanggui, WU Chongchong, ZHANG Siran. Research progress of CO2 conversion via Reverse Water-Gas Shift reaction [J]. Inorganic Chemicals Industry, 2024, 56(11): 105-115. |
| [7] | FENG Qing, WANG Yansu, ZHOU Wei, LIU Yang, SUN Yanmin, NAN Jun. Research progress on catalysts for relay-catalysis of CO2 to prepare high value-added chemicals [J]. Inorganic Chemicals Industry, 2024, 56(11): 81-94. |
| [8] | CHANG Chengbing, LIU Shengyu, ZHANG Lei, YANG Zhichao, GUO Jianying, LI Bao. Experimental study on carbon dioxide sequestration by wet carbonation of calcium silicate minerals [J]. Inorganic Chemicals Industry, 2023, 55(9): 57-65. |
| [9] | GUI Changqing, WANG Yajing, LING Changjian, WANG Huaiyou, TANG Zhongfeng. Research progress of preparation and modification of MgO-based CO2 adsorbents [J]. Inorganic Chemicals Industry, 2023, 55(8): 77-83. |
| [10] | SONG Zhijia, WANG Suisui, KUANG Qin. Hollow Cu-doped TiO2 for enhancing photocatalytic CO2 reduction performance [J]. Inorganic Chemicals Industry, 2023, 55(8): 45-50. |
| [11] | ZHAO Yan, HAO Xuewei, SHI Hainan, LI Jiahui, LI Keyan, GUO Xinwen. Study on photocatalytic CO2 reduction performance of Cu-doped TiO2/PCN heterojunction [J]. Inorganic Chemicals Industry, 2023, 55(8): 21-27. |
| [12] | WANG Jianfang, YANG Heping, LI Kaibin, CONG Shiqiang, ZHANG Bojie, GUO Shan. Study on preparation of C3N4/MnCo2S4 composites and their capacitive properties [J]. Inorganic Chemicals Industry, 2023, 55(7): 70-74. |
| [13] | LI Songhong,ZHOU Songhua,ZHAO Aiming,DONG Wenyan,JIANG Chunyan,CAO Yang,AO Xianquan. Study on catalytic gasification reaction of distillers′grains under H2O/CO2 atmosphere [J]. Inorganic Chemicals Industry, 2023, 55(2): 132-140. |
| [14] | WENG Dingsong,WANG Zhenghao,CHEN Liang,YIN Rentao,XIAO Haibing,LIU Weizao,LIANG Bin,LUO Dongmei. Serpentine coupled with copperas to enrich magnesium solution for simultaneous CO2 mineralization and nickel recovery [J]. Inorganic Chemicals Industry, 2023, 55(1): 93-99. |
| [15] | YANG Tinglong,WANG Fuzhong,LIU Fei. Study on sulfur poisoning of zirconium-based bimetallic oxides catalyst [J]. Inorganic Chemicals Industry, 2023, 55(1): 151-158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||