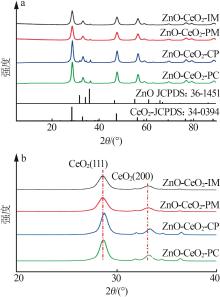
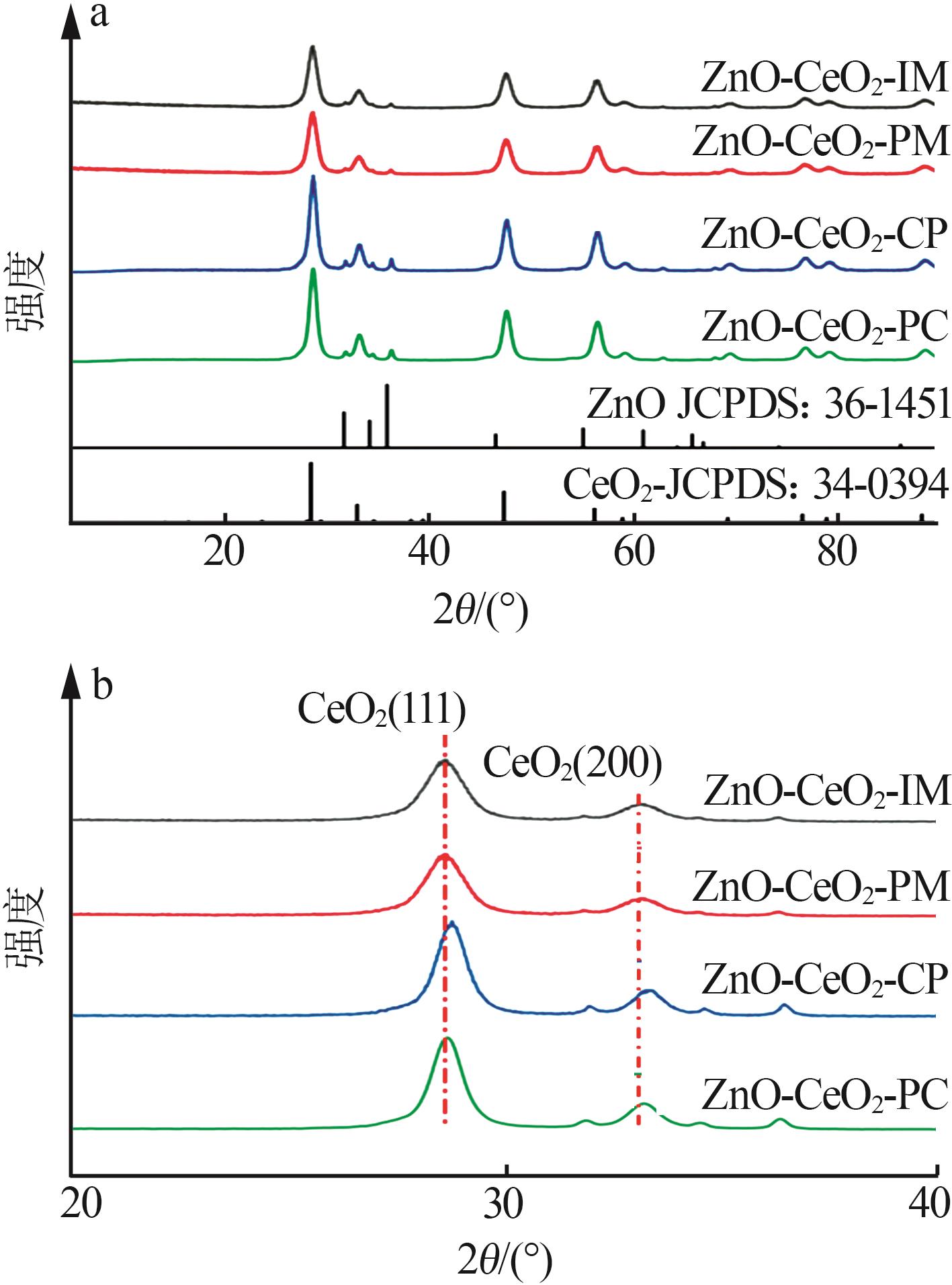
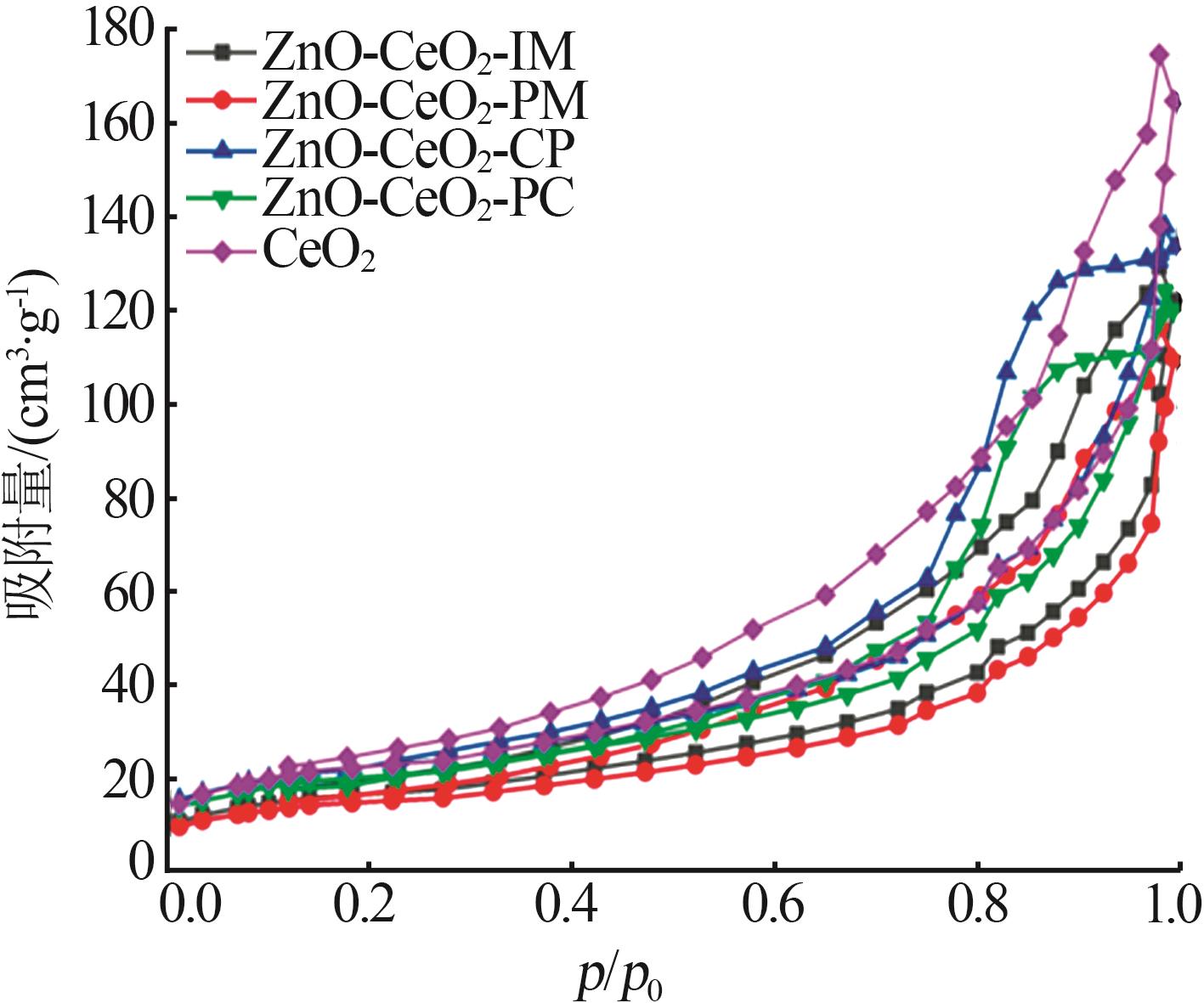
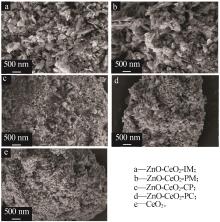
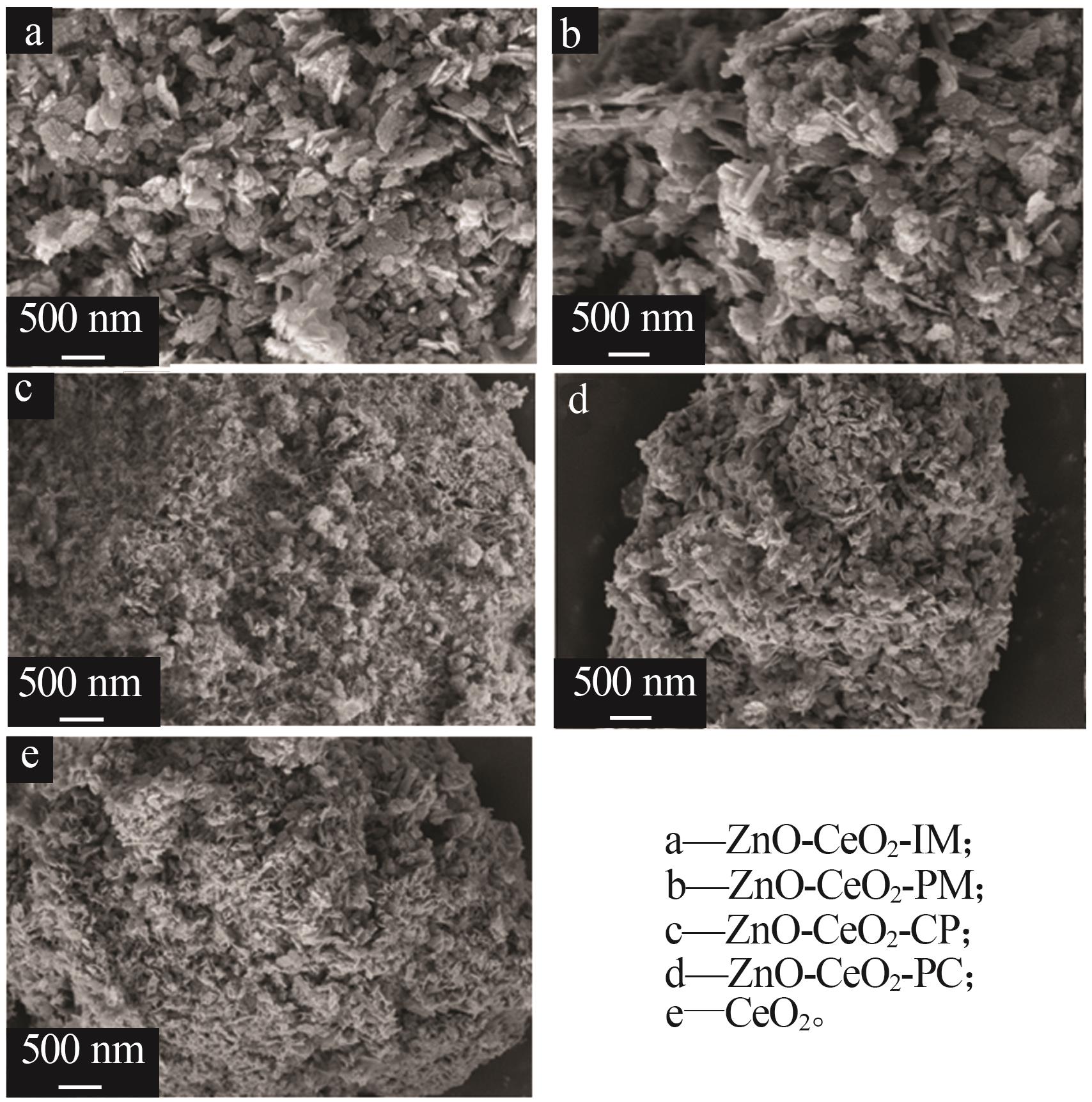
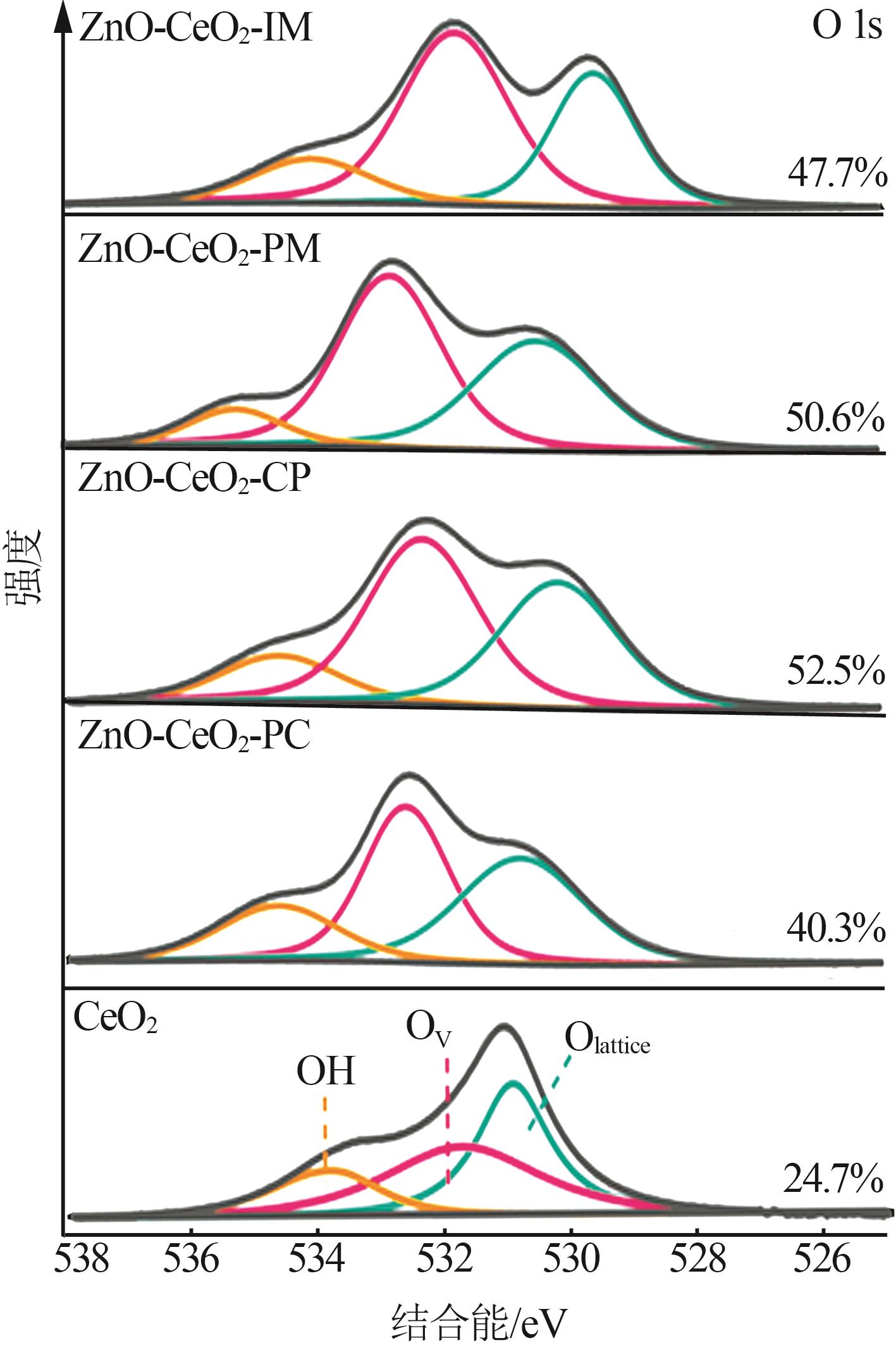
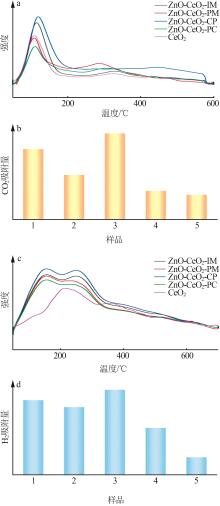
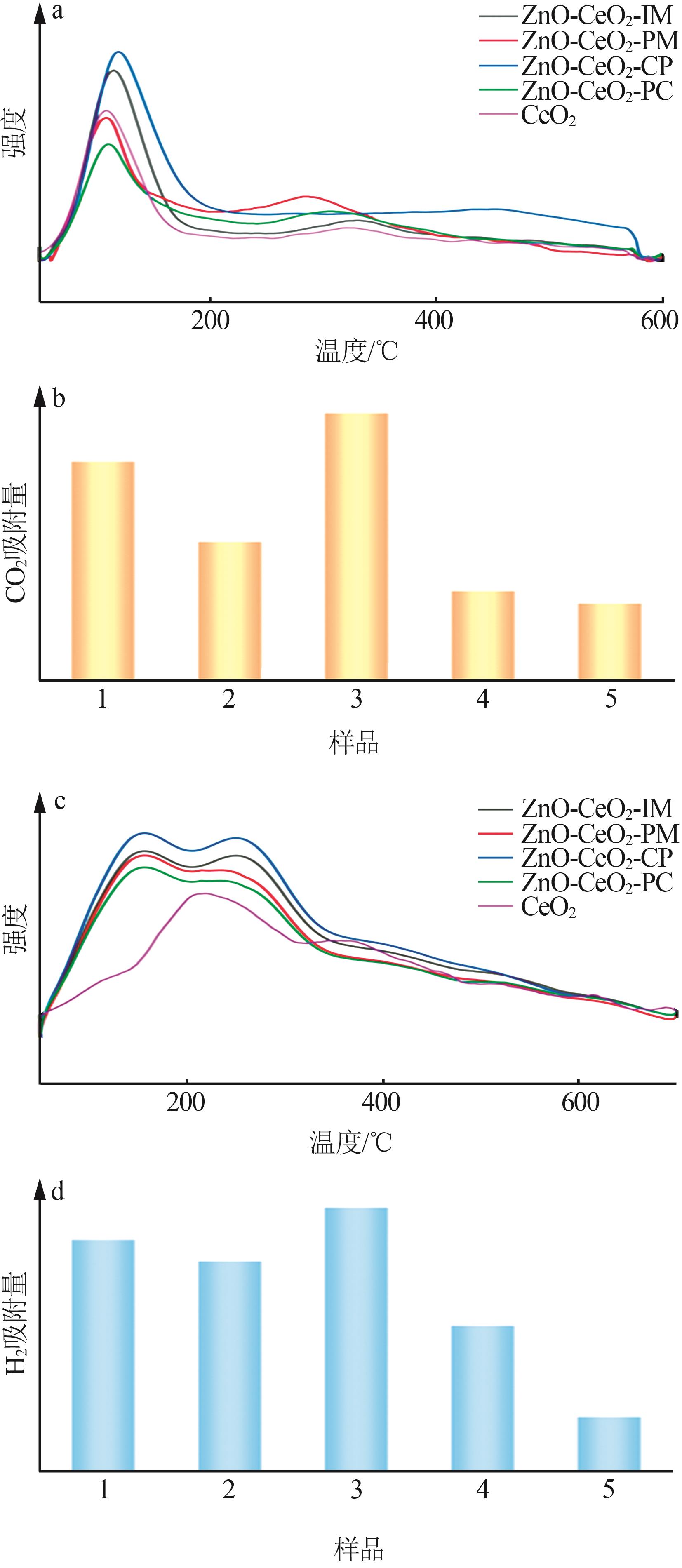
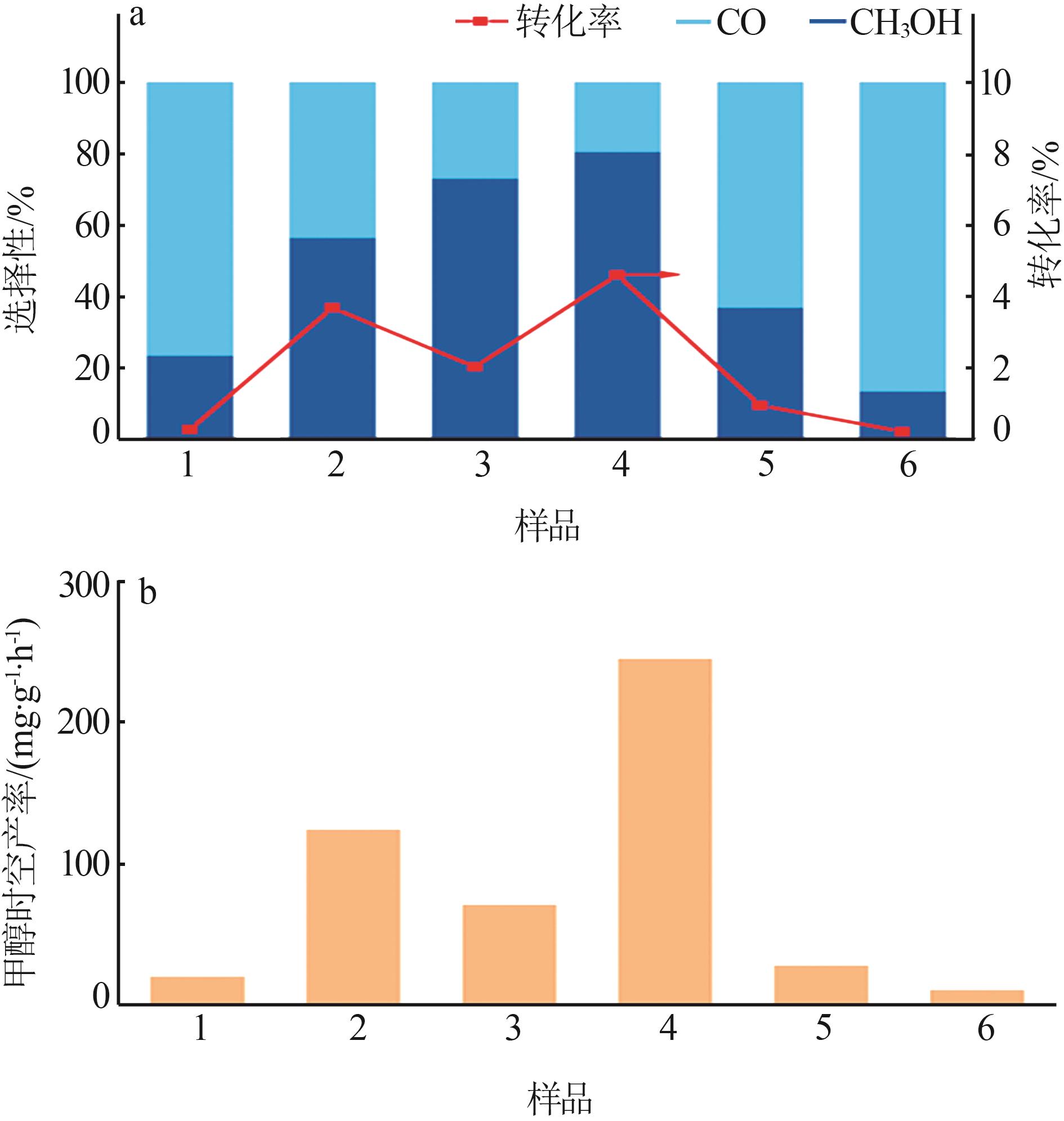
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (3): 137-143.doi: 10.19964/j.issn.1006-4990.2023-0251
• Catalytic Materials • Previous Articles Next Articles
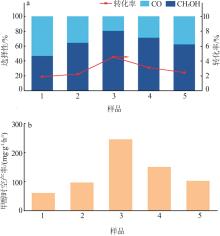
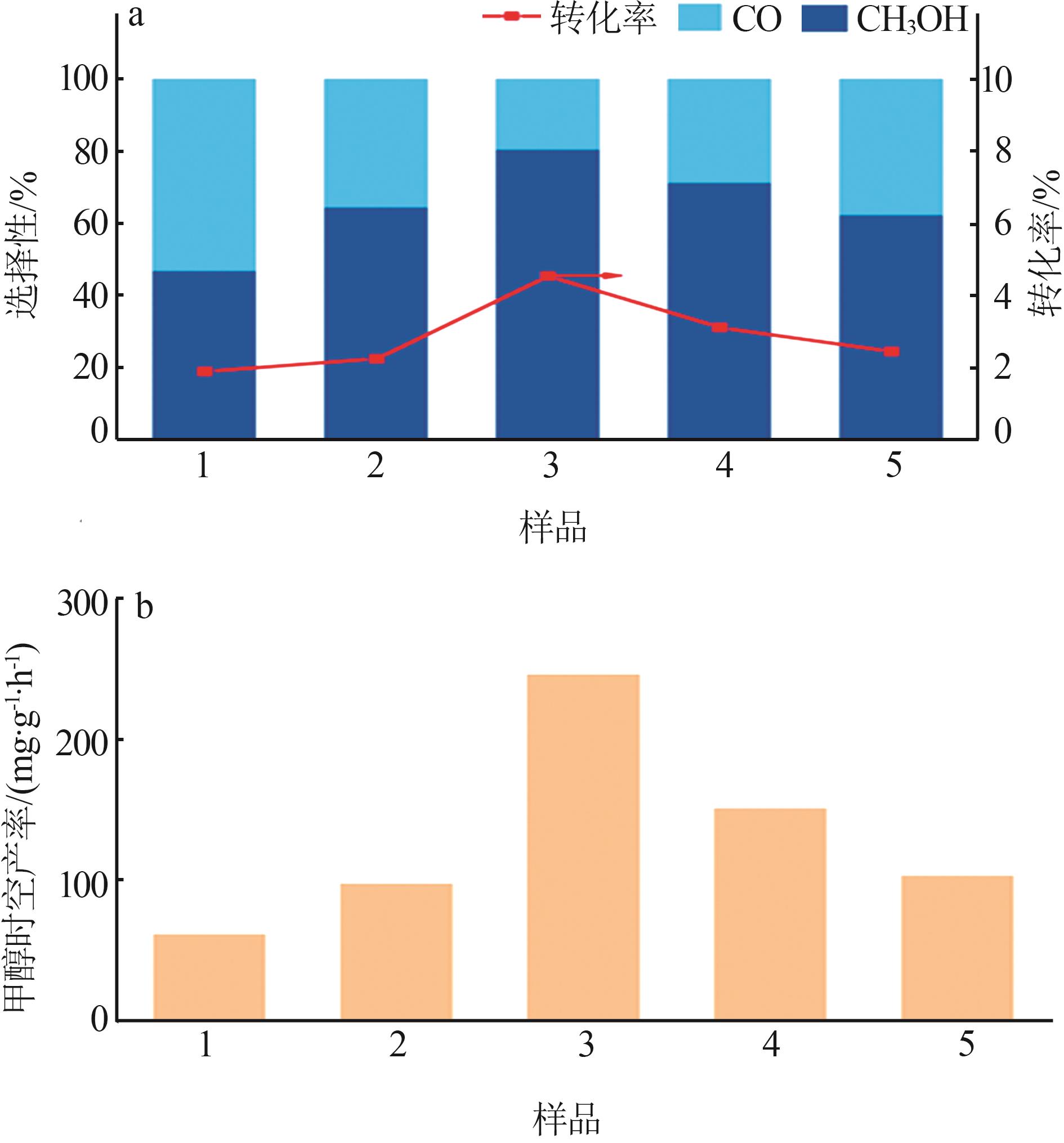
Study on preparation and catalytic performance of ZnO-CeO2
ZHAN Sijin1,2,3( ), LIU Shike1,2,3, LIU Fei1,2,3(
), LIU Shike1,2,3, LIU Fei1,2,3( ), YAO Mengqin1,2,3, CAO Jianxin1,2,3
), YAO Mengqin1,2,3, CAO Jianxin1,2,3
- 1.College of Chemistry and Chemical Engineering,Guizhou University,Guiyang 550025,China
2.Guizhou Key Laboratory for Green Chemical and Clean Energy Technology,Guiyang 550025,China
3.Guizhou Engineering Research Center of Efficient Utilization for Industrial Waste,Guiyang 550025,China
-
Received:2023-05-05Online:2024-03-10Published:2024-03-14 -
Contact:LIU Fei E-mail:457020730@qq.com;ce.feiliu@gzu.edu.cn
CLC Number:
Cite this article
ZHAN Sijin, LIU Shike, LIU Fei, YAO Mengqin, CAO Jianxin. Study on preparation and catalytic performance of ZnO-CeO2[J]. Inorganic Chemicals Industry, 2024, 56(3): 137-143.
share this article
| 1 | 张文沛.氧化亚铜改性材料的制备及其光(电)催化还原CO2的性能研究[D].武汉:华中师范大学,2015. |
| ZHANG Wenpei.Fabrication of modified cuprous oxide materials and the photoelectrocatalytic CO2 conversion to solar fuels[D].Wuhan:Central China Normal University,2015. | |
| 2 | 钟成林.二氧化碳加氢合成甲醇Cu/TiO2催化剂的研究[D].上海:上海应用技术学院,2015. |
| ZHONG Chenglin.Study of Cu/TiO2 catalysts for methanol synthesis from CO2 hydrogenation[D].Shanghai:Shanghai Institute of Technology,2015. | |
| 3 | YANG Yisen, TAN Zhonghao, WANG Sha,et al.Cu/Cu2O nanocrystals for electrocatalytic carbon dioxide reduction to multi-carbon products[J].Chemical Communications,2023,59(17):2445-2448. |
| 4 | LIU Feng, ZHANG Bo, FANG Zhi,et al.Jet-to-jet interactions in atmospheric-pressure plasma jet arrays for surface processing[J].Plasma Processes and Polymers,2018,15(1):1700114. |
| 5 | NGUYEN-PHU H, KIM T, KIM Y,et al.Role of phase in NiMgAl mixed oxide catalysts for CO2 dry methane reforming(DRM)[J].Catalysis Today,2023,411/412:113894. |
| 6 | SHARIFIANJAZI F, ESMAEILKHANIAN A, AHMADI E,et al.Methane reforming in microchannels:Application to the methanol synthesis[J].Chemical Engineering and Processing-Process Intensification,2023,185:109316. |
| 7 | AKBAR DARABADI Z ALI, MORTAZA Y, HOSSEIN N,et al.Low-carbon hydrogen,power and heat production based on steam methane reforming and chemical looping combustion[J].Energy Conversion and Management,2023,279:156-170. |
| 8 | VALLURI S, CLAREMBOUX V, KAWATRA S.Opportunities and challenges in CO2 utilization[J].Journal of Environmental Scien- ces,2022,113:322-344. |
| 9 | CHANG Kuan, ZHANG Haochen, CHENG M J,et al.Application of ceria in CO2 conversion catalysis[J].ACS Catalysis,2020,10(1):613-631. |
| 10 | YUAN Jing, YANG Manping, HU Qiaoli,et al.Cu/TiO2 nanoparticles modified nitrogen-doped graphene as a highly efficient catalyst for the selective electroreduction of CO2 to different alcohols[J].Journal of CO2 Utilization,2018,24:334-340. |
| 11 | SZANIAWSKA E, BIENKOWSKI K, RUTKOWSKA I A,et al.Enhanced photoelectrochemical CO2-reduction system based on mixed Cu2O-nonstoichiometric TiO2 photocathode[J].Catalysis Today,2018,300:145-151. |
| 12 | BAO Yunfeng, HUANG Chunlei, CHEN Limin,et al.Highly efficient Cu/anatase TiO2{001}-nanosheets catalysts for methanol synthesis from CO2 [J].Journal of Energy Chemistry,2018,27(2):381-388. |
| 13 | ENSAFI A A, ALINAJAFI H A, REZAEI B.Pt-modified nitrogen doped reduced graphene oxide:A powerful electrocatalyst for direct CO2 reduction to methanol[J].Journal of Electroanalytical Chemistry,2016,783:82-89. |
| 14 | 谢易欣.含过渡金属(M=Pt,Au,Cu,Zn)/石墨烯复合催化剂的设计合成及其电(光)催化性能[D].福州:福建师范大学,2019. |
| XIE Yixin.Design,synthesis and electrical(photo)catalytic performance of transition metal-containing(M=Pt,Au,Cu,Zn)/graphene composite catalysts[D].Fuzhou:Fujian Normal University,2019. | |
| 15 | WANG Xilong, ALABSI M H, ZHENG Peng,et al.PdCu supported on dendritic mesoporous Ce x Zr1- x O2 as superior catalysts to boost CO2 hydrogenation to methanol[J].Journal of Colloid and Interface Science,2022,611:739-751. |
| 16 | NIU Juntian, LIU Haiyu, JIN Yan,et al.Comprehensive review of Cu-based CO2 hydrogenation to CH3OH:Insights from experimental work and theoretical analysis[J].International Journal of Hydrogen Energy,2022,47(15):9183-9200. |
| 17 | 张平,陈浩,陈林,等.基于NiZn层状双金属氢氧化物制备高效电催化CO2还原的原子分散Ni-N-C催化剂[J].催化学报(英文),2023,44(2):152-161. |
| ZHANG Ping, CHEN Hao, CHEN Lin,et al.Atomically dispersed Ni-N-C catalyst derived from NiZn layered double hydroxides for efficient electrochemical CO2 reduction[J].Chinese Journal of Catalysis,2023,44(2):152-161. | |
| 18 | SU Haiyan, MA Xiufang, SUN Chenghua,et al.A synergetic effect between a single Cu site and S vacancy on an MoS2 basal plane for methanol synthesis from syngas[J].Catalysis Science & Technology,2021,11(9):3261-3269. |
| 19 | PACHOLIK G, ENZLBERGER L, BENZER A,et al. in situ XPS studies of MoS2-based CO2 hydrogenation catalysts[J].Journal of Physics D:Applied Physics,2021,54(32):324002. |
| 20 | HU Jingting, YU Liang, DENG Jiao,et al.Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol[J].Nature Catalysis,2021,4(3):242-250. |
| 21 | CUI Pingping, SUN Ruyu, XIAO Linfei,et al.Exploring the effects of the interaction of carbon and MoS2 catalyst on CO2 hydrogenation to methanol[J].International Journal of Molecular Sciences,2022,23(9):5220. |
| 22 | WANG Jijie, LI Guanna, LI Zelong,et al.A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol[J].Science Advances,2017,3(10):e1701290. |
| 23 | WANG Jijie, TANG Chizhou, LI Guanna,et al.High-performance MaZrO x (Ma=Cd,Ga) solid-solution catalysts for CO2 hydrogenation to methanol[J].ACS Catalysis,2019,9(11):10253-10259. |
| 24 | WANG Weiwei, QU Zhenping, SONG Lixin,et al.CO2 hydrogenation to methanol over Cu/CeO2 and Cu/ZrO2 catalysts:Tuning methanol selectivity via metal-support interaction[J].Journal of Energy Chemistry,2020,40:22-30. |
| 25 | ZHANG Wenyu, WANG Sen, GUO Shujia,et al.Effective conversion of CO2 into light olefins along with generation of low amoun-ts of CO[J].Journal of Catalysis,2022,413:923-933. |
| 26 | TADA S, OCHIAI N, KINOSHITA H,et al.Active sites on Zn x Zr1– x O2– x solid solution catalysts for CO2-to-methanol hydrogenation[J].ACS Catalysis,2022,12(13):7748-7759. |
| [1] | CHENG Xiaoqiang, MA Jun, FENG Bin, BAI Liguang, ZHAO Xiaodong. Discussion on application of post-treatment process in fixed bed hydrogen peroxide production process [J]. Inorganic Chemicals Industry, 2025, 57(2): 98-104. |
| [2] | LI Yongheng, MI Xiaotong, ZHANG Peipei. Synthesis of holycrystalline nano-ZSM-5 aggregate and its application in methylation of toluene [J]. Inorganic Chemicals Industry, 2024, 56(5): 135-140. |
| [3] | REN Qixia, YANG Kun, LIU Fei, YAO Mengqin, CAO Jianxin. Effect of promoter on physicochemical properties and catalytic performance of ZnO/ZrO2 [J]. Inorganic Chemicals Industry, 2024, 56(3): 144-154. |
| [4] | YANG Kun, REN Qixia, DONG Yonggang, LIU Fei, YAO Mengqin, CAO Jianxin. Effect of calcination temperature on catalytic performance of ZnGaZrO x /SAPO-34 [J]. Inorganic Chemicals Industry, 2024, 56(2): 136-145. |
| [5] | LI He, ZHANG Lijie, ZHANG Kai, SU Jin, YAO Zhaoyang, ZENG Xianjun, GUO Chunlei, SUN Yanmin. Advances in technology and catalyst for methanol oxidized to formaldehyde [J]. Inorganic Chemicals Industry, 2023, 55(11): 12-18. |
| [6] | YANG Tinglong,WANG Fuzhong,LIU Fei. Study on sulfur poisoning of zirconium-based bimetallic oxides catalyst [J]. Inorganic Chemicals Industry, 2023, 55(1): 151-158. |
| [7] | CHEN Jun,ZHONG Jing,LIN Sen,YU Jianguo. Study on lithium separation from brine by aluminum-based adsorbent in fixed bed [J]. Inorganic Chemicals Industry, 2023, 55(1): 64-73. |
| [8] | JI Chao,WU Luming,LI Bin,ZANG Jiazhong,YU Haibin. Research progress on modification of SAPO-34 as catalyst for methanol to olefins [J]. Inorganic Chemicals Industry, 2022, 54(7): 1-9. |
| [9] | Peng Xiaowei,Wang Yinbin,Zang Jiazhong,Yu Haibin. Research on preparation of metal modified catalysts and catalytic performances of methanol aromatization [J]. Inorganic Chemicals Industry, 2021, 53(9): 104-108. |
| [10] | Xiao Yihan,Cao Jianxin,Liu Fei,Yi Yun. Effect of calcination temperature on physicochemical properties and catalytic performance of MnZnOx [J]. Inorganic Chemicals Industry, 2021, 53(4): 95-100. |
| [11] | Li Xiaoguo, Li Yongheng, Hou Zhanggui, Han Guodong, Xiao Jiawang, Gao Jinping, Chang Yang. Study on pilot catalytic performance of the modified catalyst for alkylation of toluene with methanol to p-xylene [J]. Inorganic Chemicals Industry, 2021, 53(3): 97-101. |
| [12] | Hou Zhanggui,Zhu Qianqian,Li Xiaoguo,Li Yongheng,Chang Yang,Zhang Anfeng,Guo Xinwen. Preparation of modificated micro-mesoporous ZSM-5 molecular sieve catalyst and its catalytic performance for alkylation of toluene with methanol [J]. Inorganic Chemicals Industry, 2020, 52(9): 96-99. |
| [13] | Li Xiangguo,Guo Miao,Yao Huimin,Lü Jing. Research on hydrothermal synthesis and photoluminescence mechanism of rhombus CeO2 [J]. Inorganic Chemicals Industry, 2020, 52(6): 30-35. |
| [14] | Su Wei,Zhang Linfeng,Lü Qingyang,Xia Yuyan,Yuan Hua. Synthesis of diphenyl carbonate by oxidative carbonylation over palladium loaded MnOx-based bimetallic oxide catalysis [J]. Inorganic Chemicals Industry, 2019, 51(9): 91-96. |
| [15] | Liu Zhikai1,Cao Hui2,Wang Tianyun1,Han Guodong2,Liu Yi1,Zhang Yi1. Study on novel catalysts for direct catalytic conversion of unconventional natural gas to aromatics [J]. Inorganic Chemicals Industry, 2019, 51(12): 83-88. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||