Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (6): 14-22.doi: 10.19964/j.issn.1006-4990.2021-0236
• Inorganic Noval Materials—Energy Storage and Conversion • Previous Articles Next Articles
Applications of titanium-based compounds for lithium-sulfur batteries
Liu Zichen( ),Zhang bin,Gu Sichen(
),Zhang bin,Gu Sichen( ),Lü Wei
),Lü Wei
- Tsinghua Shenzhen International Graduate School,Tsinghua University,Shenzhen 518000,China
-
Received:2021-04-20Online:2021-06-10Published:2021-07-08 -
Contact:Gu Sichen E-mail:liuzc19@mails.tsinghua.edu.cn;sichen.gu@sz.tsinghua.edu.cn
CLC Number:
Cite this article
Liu Zichen,Zhang bin,Gu Sichen,Lü Wei. Applications of titanium-based compounds for lithium-sulfur batteries[J]. Inorganic Chemicals Industry, 2021, 53(6): 14-22.
share this article
Table 1
Electrochemical performance of titanium-based compound lithium-sulfur battery"
| 正极添加剂/中间层材料 | 倍率(C) | 初始容量/ (mA·h·g-1) | 循环 寿命/次 | 容量 衰减率/% | 硫载量/ (mg·cm-2) | |
|---|---|---|---|---|---|---|
| 氧化物 | TiO2-x球壳/S正极[ | 0.2 | 1 100 | 200 | 0.095 | 0.8 |
| S@TiO2-x多层壳正极[ | 0.5 | 903 | 1 000 | 0.021 | 0.5 | |
| “蛋黄-蛋壳”结构S-TiO2正极[ | 0.5 | 1 030 | 1 000 | 0.033 | 0.4~0.6 | |
| TiO@C/S正极[ | 0.5 | 1 066 | 500 | 0.082 | 1.5 | |
| SCM(介孔碳)/S-αTiO2正极[ | 1.0 | 1 201 | 200 | 0.135 | 0.75 | |
| S@TiO2/聚吡咯纳米线正极[ | 1.0 | 900 | 500 | 0.098 | — | |
| Ti4O7/S正极[ | 2.0 | 850 | 500 | 0.060 | 0.75~0.9 | |
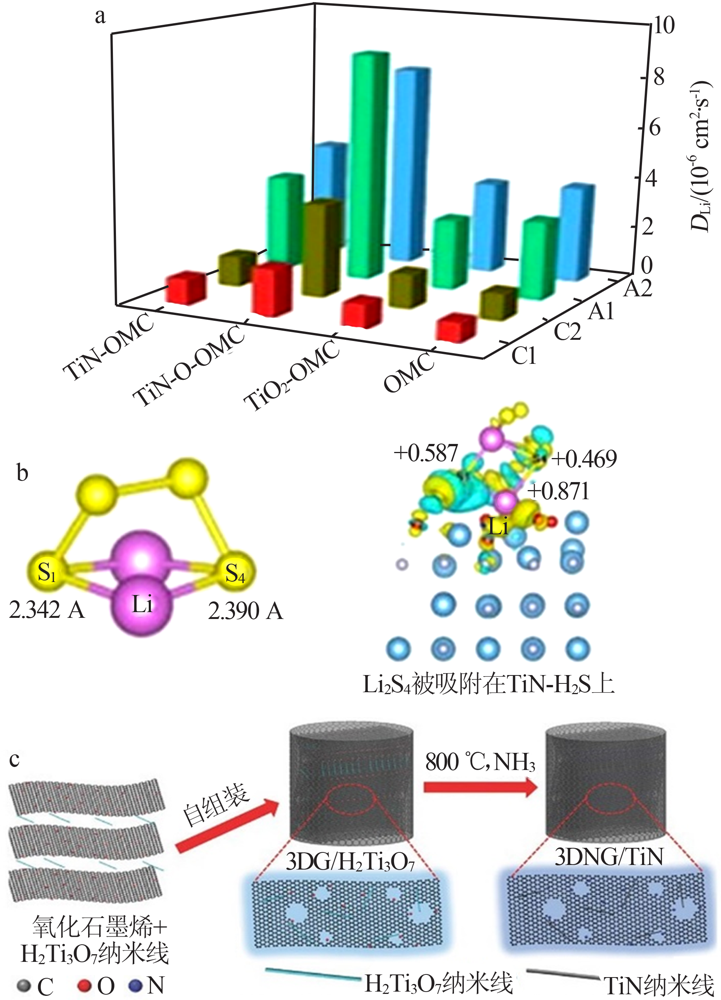
| 氮化物 | 介孔TiN/S[ | 0.5 | 988 | 500 | 0.070 | 1.0 |
| 氢氟酸刻蚀TiN/S正极[ | 0.5 | 1 017 | 200 | 0.175 | 1.5 | |
| 3DNG/TiN/Li2S6正极[ | 0.5 | 1 250 | 60 | 0.280 | 9.6 | |
| TiN-H2S中间层[ | 1.0 | 480 | 500 | 0.039 | 3.4 | |
| TiN-O-有序介孔碳(OMC)/S正极材料[ | 5.0 | 726 | 120 | 0.064 | 1.4 | |
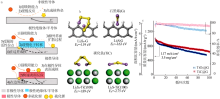
| 碳化物 | TiC@G/S正极[ | 0.2 | 1 032 | 100 | 0.350 | 3.5 |
| S/TiC-CNFs正极[ | 1.0 | 1 079 | 350 | 0.120 | 1.0 | |
| 异质结构 | Ni3S2-TiO2异质结构/石墨烯/S正极[ | 0.5 | 980 | 900 | 0.004 | — |
| TiO2-TiN异质结构中间层[ | 1.0 | 688 | 2 000 | 0.013 | 3.1 | |
| 石墨烯-TiC异质结构中间层[ | 1.0 | 560 | 500 | 0.032 | 1.1~1.4 | |

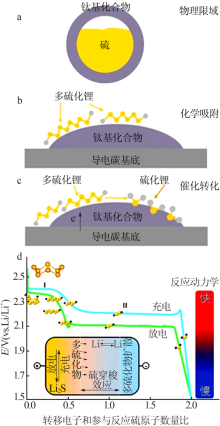
Fig.4
(a) FTIR spectra and(b) Raman spectra of neat α-TiO2, Li2S4,and α-TiO2/Li2S4[31];Adsorption models of the S42- upon the(001) crystal plane of the anatase TiO2(c)[41];Schematic of the synthetic process and characterization of sulfur-TiO2 yolk-shell nanostructures and SEM images(d)[10];Schematic diagram of the preparation process of TiO2-x with hollow spherical shell structure(e)[28] "
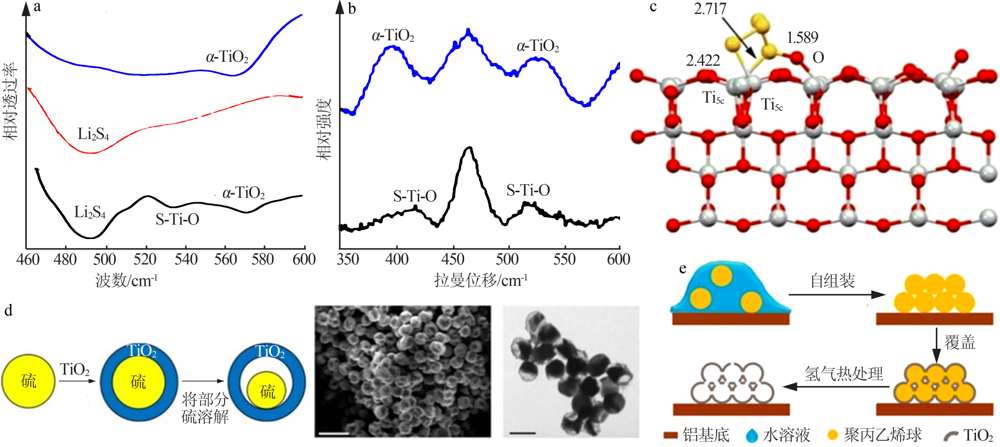
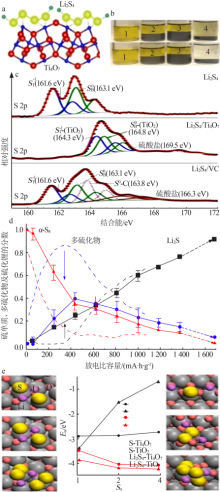
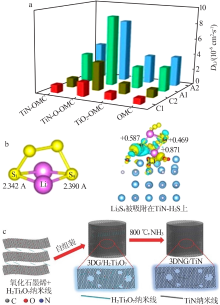
Fig.5
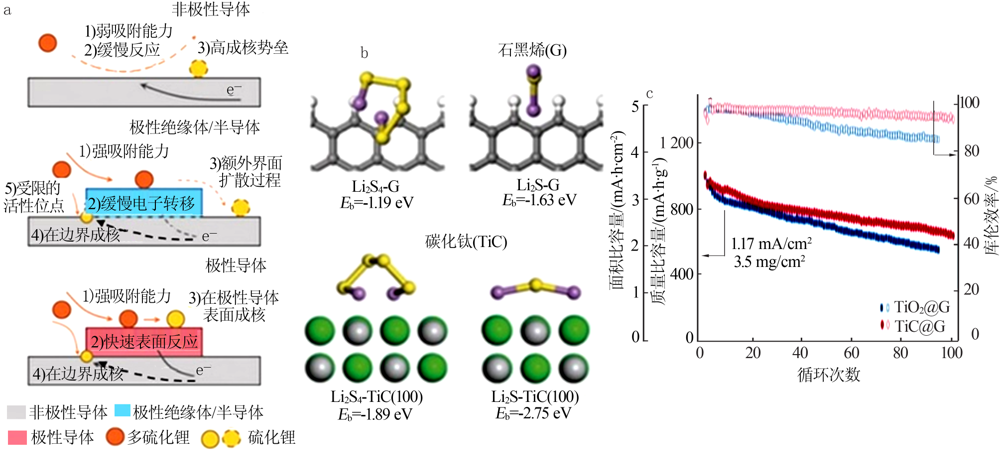
Schematic showing the electron density transfer bet-ween Li2S4 and TiOx(a);Lithium polysulfide adsorption test:1—Li2S4 solution with nothing;2—with graphite;3—with VC carbon;4—with Ti4O7(b);XPS S 2p spectra of Li2S4,Li2S4/VC carbon and Li2S4/Ti4O7(c);Distribution of sulphur species upon discharge determined by operando XANES(d)[23];DFT analysis of the adsorption of S species on TiO2(110) and Ti4O7(1-21) surfaces(e)[48] "
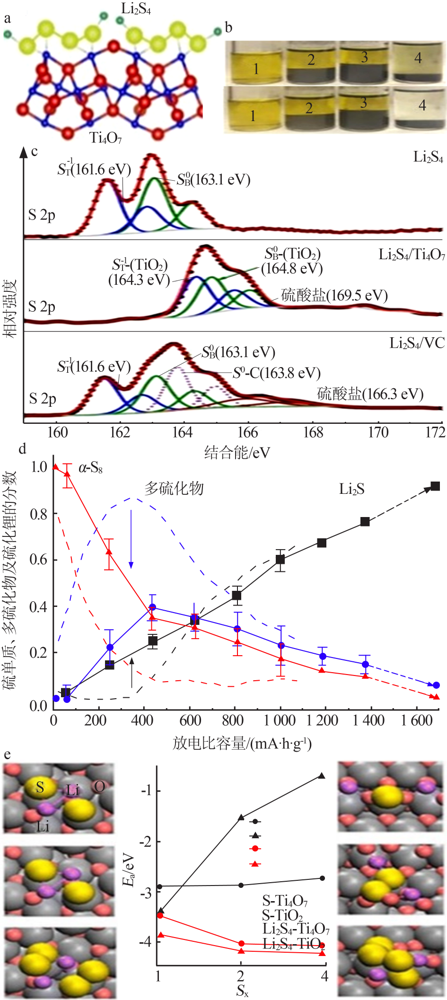
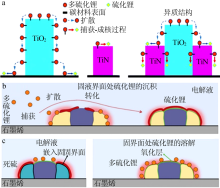
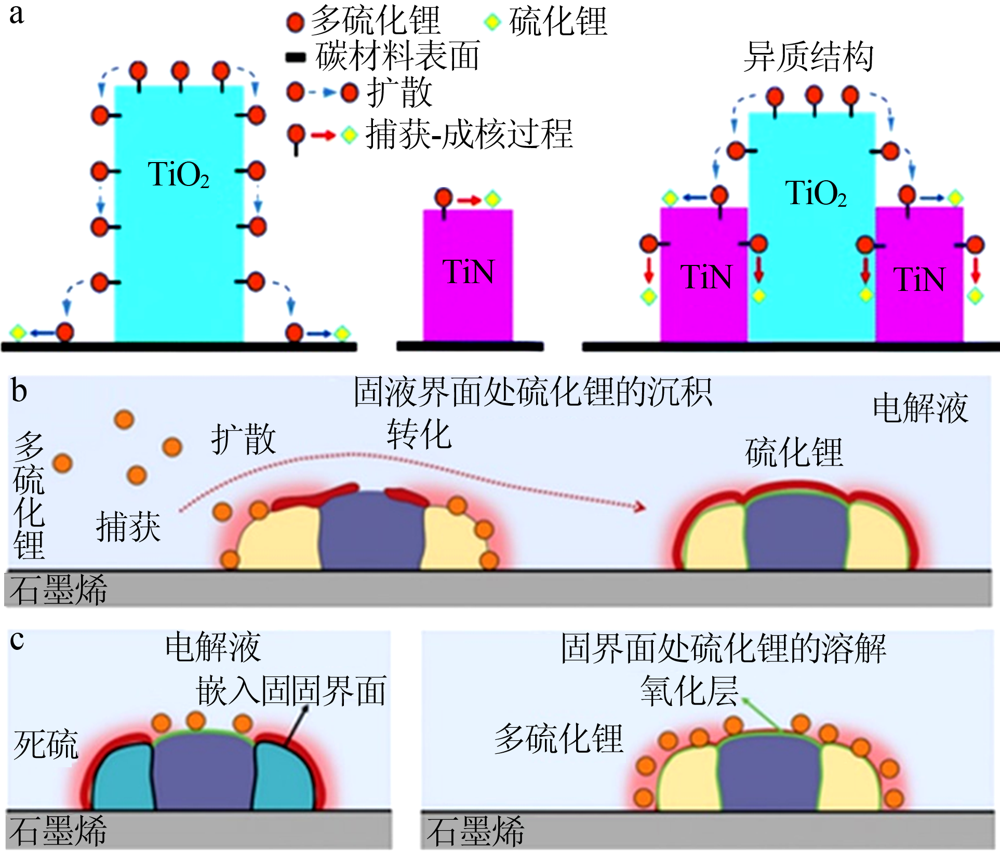
| [1] | Pang Q, Liang X, Kwok C Y, et al. Advances in lithium-sulfur batte-ries based on multifunctional cathodes and electrolytes[J]. Nature Energy, 2016,1(9).Doi: 10.1038/nenergy.2016.132. |
| [2] |
Bruce P G, Freunberger S A, Hardwick L J, et al. Li-O2 and Li-S bat-teries with high energy storage[J]. Nature Materials, 2011,11(1):19-29.
doi: 10.1038/nmat3191 |
| [3] |
Manthiram A, Fu Y, Chung S H, et al. Rechargeable lithium-sulfur batteries[J]. Chemical Reviews, 2014,114(23):11751-11787.
doi: 10.1021/cr500062v |
| [4] |
Goodenough J B. Electrochemical energy storage in a sustainable mo-dern society[J]. Energy Environmental Science, 2014,7(1):14-18.
doi: 10.1039/C3EE42613K |
| [5] |
Zhang C, Cui L, Abdolhosseinzadeh S, et al. Two-dimensional mxenes for lithium-sulfur batteries[J]. Info.Mat., 2020,2(4):613-638.
doi: 10.1002/inf2.v2.4 |
| [6] | Liu D, Zhang C, Zhou G, et al. Catalytic effects in lithium-sulfur bat-teries:Promoted sulfur transformation and reduced shuttle effect[J]. Advanced Science, 2018,5(1).Doi: 10.1002/advs. 201700270. |
| [7] | Liu X, Huang J Q, Zhang Q, et al. Nanostructured metal oxides and sulfides for lithium-sulfur batteries[J]. Advanced Materials, 2017,29(20).Doi: 10.1002/adma.201601759. |
| [8] | Zhang Z W, Peng H J, Zhao M, et al. Heterogeneous/homogeneous mediators for high-energy-density lithium-sulfur batteries:Progress and prospects[J]. Advanced Functional Materials, 2018,28(38).Doi: 10.1002/adfm.201707536. |
| [9] | Mei S, Jafta C J, Lauermann I, et al. Porous Ti4O7 particles with in terconnected-pore structure as a high-efficiency polysulfide mediator for lithium-sulfur batteries[J]. Advanced Functional Materials, 2017,27(26).Doi: 10.1002/adfm.201701176. |
| [10] | Wei Seh Z, Li W, Cha J J, et al. Sulphur-TiO2 yolk-shell nanoarchi-tecture with internal void space for long-cycle lithium-sulphur bat-teries[J]. Nature Communications, 2013(4).Doi: 10.1038/ncomms2327. |
| [11] | Shi H, Lv W, Zhang C, et al. Functional carbons remedy the shuttl-ing of polysulfides in lithium-sulfur batteries:Confining,trapping,blocking,and breaking up[J]. Advanced Functional Materials, 2018,28(38).Doi: 10.1002/adfm.201800508. |
| [12] | Lee B J, Kang T H, Lee H Y, et al. Revisiting the role of conductivi-ty and polarity of host materials for long-life lithium-sulfur bat-tery[J]. Advanced Energy Materials, 2020,10(22).Doi: 10.1002/aenm.201903934. |
| [13] | Ye C, Jiao Y, Jin H, et al. 2D MoN-VN heterostructure to regulate polysulfides for highly efficient lithium-sulfur batteries[J]. Ange-wandte Chemie:International Edition, 2018,57(51):16703-16707. |
| [14] |
Chung S H, Luo L, Manthiram A. TiS2-polysulfide hybrid cathode with high sulfur loading and low electrolyte consumption for lithi-um-sulfur batteries[J]. ACS Energy Letters, 2018,3(3):568-573.
doi: 10.1021/acsenergylett.7b01321 |
| [15] |
Zhou F, Li Z, Luo X, et al. Low cost metal carbide nanocrystals as binding and electrocatalytic sites for high performance Li-S bat-batteries[J]. Nano Letters, 2018,18(2):1035-1043.
doi: 10.1021/acs.nanolett.7b04505 |
| [16] | Wang H, Zhang W, Xu J, et al. Advances in polar materials for lit-hium-sulfur batteries[J]. Advanced Functional Materials, 2018,28(38).Doi: 10.1002/adfm.201707520. |
| [17] | 罗远辉. 钛化合物[M]. 北京: 冶金工业出版社, 2011:1-7. |
| [18] |
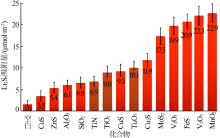
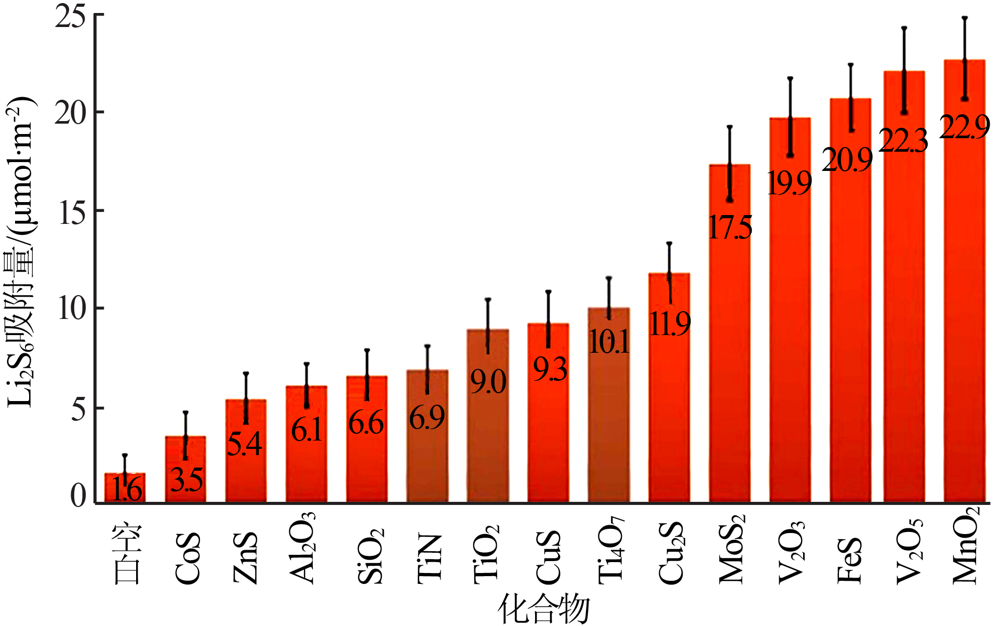
Wu D S, Shi F, Zhou G, et al. Quantitative investigation of polysul-fide adsorption capability of candidate materials for Li-S batteri-es[J]. Energy Storage Materials, 2018,13:241-246.
doi: 10.1016/j.ensm.2018.01.020 |
| [19] | 黄仲涛. 工业催化剂手册[M]. 北京: 化学工业出版社, 2004:17-31. |
| [20] | 日本钛协会. 钛材料及其应用[M].周连在,译.1版. 北京: 冶金工业出版社, 2008:1-25. |
| [21] | Zhou G, Tian H, Jin Y, et al. Catalytic oxidation of Li2S on the sur-face of metal sulfides for Li-S batteries[J]. Proceedings of the Na-tional Academy of Sciences of the United States of America, 2017,114(5):840-845. |
| [22] |
Zhang Q F, Wang Y P, Seh Z W, et al. Understanding the anchoring effect of two-dimensional layered materials for lithium-sulfur bat-teries[J]. Nano Letters, 2015,15(6):3780-3786.
doi: 10.1021/acs.nanolett.5b00367 |
| [23] | Pang Q, Kundu D, Cuisinier M, et al. Surface-enhanced redox che-mistry of polysulphides on a metallic and polar host for lithium-sulphur batteries[J]. Nature Communications, 2014(5).Doi: 10.1038/ncomms5759. |
| [24] |
Peng H J, Zhang G, Chen X, et al. Enhanced electrochemical kine-tics on conductive polar mediators for lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2016,55(42):12990-12995.
doi: 10.1002/anie.201605676 |
| [25] | Li Z H, He Q, Xu X, et al. A 3D nitrogen-doped graphene/TiN nano-wires composite as a strong polysulfide anchor for lithium-sulfur batteries with enhanced rate performance and high areal capaci-ty[J]. Advanced Materials, 2018,30(45).Doi: 10.1002/adma. |
| 201804089. | |
| [26] | Tao X, Wang J, Liu C, et al. Balancing surface adsorption and diffu-sion of lithium-polysulfides on nonconductive oxides for lithium-sulfur battery design[J]. Nature Communications, 2016,7.Doi: 10.1038/ncomms.11203. |
| [27] | Wang D W, Zeng Q, Zhou G, et al. Carbon-sulfur composites for Li-S batteries:Status and prospects[J]. Journal of Materials Che-mistry A, 2013,1(33):9382-9394 |
| [28] |
Liang Z, Zheng G Y, Li W Y, et al. Sulfur cathodes with hydrogen reduced titanium dioxide inverse opal structure[J]. ACS Nano, 2014,8(5):5249-5256.
doi: 10.1021/nn501308m pmid: 24766547 |
| [29] |
Salhabi E H M, Zhao J, Wang J, et al. Hollow multi-shelled struc-tural TiO2-x with multiple spatial confinement for long-life lithium-sulfur batteries[J]. Angewandte Chemie:International Edition, 2019,58(27):9078-9082.
doi: 10.1002/anie.v58.27 |
| [30] | Li Z, Zhang J, Guan B, et al. A sulfur host based on titanium mono-xide@carbon hollow spheres for advanced lithium-sulfur batteri-es[J]. Nature Communications, 2016(7).Doi: 10.1038/ncomms. 13065. |
| [31] |
Evers S, Yim T, Nazar L F. Understanding the nature of absorption/adsorption in nanoporous polysulfide sorbents for the Li-Sbattery[J]. The Journal of Physical Chemistry C, 2012,116(37):19653-19658.
doi: 10.1021/jp304380j |
| [32] |
Wu J, Li S, Yang P, et al. S@TiO2 nanospheres loaded on PPy ma-trix for enhanced lithium-sulfur batteries[J]. Journal of Alloys and Compounds, 2019,783:279-285
doi: 10.1016/j.jallcom.2018.12.316 |
| [33] | Cui Z M, Zu C X, Zhou W D, et al. Mesoporous titanium nitride-enabled highly stable lithium-sulfur batteries[J]. Advanced Mate-rials, 2016,28(32):6926-6931. |
| [34] | Hao Z, Yuan L, Chen C, et al. TiN as a simple and efficient poly-sulfide immobilizer for lithium-sulfur batteries[J]. Journal of Ma-terials Chemistry A, 2016,4(45):17711-17717. |
| [35] |
Hao B, Li H, Lv W, et al. Reviving catalytic activity of nitrides by the doping of the inert surface layer to promote polysulfide conver-sion in lithium-sulfur batteries[J]. Nano Energy, 2019,60:305-311.
doi: 10.1016/j.nanoen.2019.03.064 |
| [36] |
Gao X, Zhou D, Chen Y, et al. Strong charge polarization effect en- abled by surface oxidized titanium nitride for lithium-sulfur bat- teries[J]. Communications Chemistry, 2019,2(1):66.
doi: 10.1038/s42004-019-0166-8 |
| [37] |
Zhou F, Song L T, Lu L L, et al. Titanium-carbide-decorated carbon nanofibers as hybrid electrodes for high performance Li-S batteri- es[J]. ChemNanoMat, 2016,2(10):937-941.
doi: 10.1002/cnma.201600227 |
| [38] | Wang R, Luo C, Wang T, et al. Bidirectional catalysts for liquid-so- lid redox conversion in lithium-sulfur batteries[J]. Advanced Ma- terials, 2020,32(32).Doi: 10.1002/adma.202000315. |
| [39] | Zhou T, Lv W, Li J, et al. Twinborn TiO2-TiN heterostructures en- abling smooth trapping-diffusion-conversion of polysulfides towar- ds ultralong life lithium-sulfur batteries[J]. Energy & Environmen- tal Science, 2017,10(7):1694-1703. |
| [40] |
Zhou T, Zhao Y, Zhou G, et al. An in-plane heterostructure of grap- hene and titanium carbide for efficient polysulfide confinement[J]. Nano Energy, 2017,39:291-296.
doi: 10.1016/j.nanoen.2017.07.012 |
| [41] | Yuan C, Zhu S, Cao H, et al. Hierarchical sulfur-impregnated hy- drogenated TiO2 mesoporous spheres comprising anatase nanoshee- ts with highly exposed(001) facets for advanced Li-S batteries[J]. Nanotechnology, 2016,27(4).Doi: 10.1088/0957-4484/27/4/045403. |
| [42] | Ma X Z, Jin B, Wang H Y, et al. S-TiO2 composite cathode materi- als for lithium/sulfur batteries[J]. Journal of Electroanalytical Che- mistry, 2015,736:127-131. |
| [43] |
Li Q, Zhang Z A, Zhang K, et al. Synjournal and electrochemical performance of TiO2-sulfur composite cathode materials for lithi- um-sulfur batteries[J]. Journal of Solid State Electrochemistry, 2013,17(11):2959-2965.
doi: 10.1007/s10008-013-2203-3 |
| [44] |
Xie Keyu, Han Yunzhao, Wei Wenfei, et al. Fabrication of a novel TiO2/S composite cathode for high performance lithium-sulfur bat- teries[J]. RSC Advances, 2015,5(94):77348-77353.
doi: 10.1039/C5RA13823J |
| [45] | Lei T, Xie Y, Wang X, et al. TiO2 feather duster as effective polysul- fides restrictor for enhanced electrochemical kinetics in lithium- sulfur batteries[J]. Small, 2017,13(37).Doi: 10.1002/smll.201701013. |
| [46] |
Nowotny J, Alim M A, Bak T, et al. Defect chemistry and defect en- gineering of TiO2-based semiconductors for solar energy conver- sion[J]. Chemical Society Reviews, 2015,44(23):8424-8442.
doi: 10.1039/c4cs00469h pmid: 26446476 |
| [47] |
Naldoni A, Allieta M, Santangelo S, et al. Effect of nature and loc- ation of defects on bandgap narrowing in black TiO2 nanoparticl- es[J]. Journal of the American Chemical Society, 2012,134(18):7600-7603.
doi: 10.1021/ja3012676 |
| [48] |
Tao X Y, Wang J G, Ying Z G, et al. Strong sulfur binding with con- ducting magnéli-phase TinO2n-1 nanomaterials for improving lithi- um-sulfur batteries[J]. Nano Letters, 2014,14(9):5288-5294.
doi: 10.1021/nl502331f |
| [49] | Zhang Miao, Chen Wei, Xue LanXin, et al. Adsorption-catalysis design in the lithium-sulfur battery[J]. Advanced Energy Materi- als, 2020,10(2):Doi: 10.1002/aenm.201903008. |
| [50] | Huang S, Wang Z, Von Lim Y, et al. Recent advances in heterost- structure engineering for lithium-sulfur batteries[J]. Advanced Energy Materials, 2021,11(10).Doi: 10.1002/aenm.202003689. |
| [51] | Zhao M, Li B, Peng H, et al. Lithium-sulfur batteries under lean electrolyte conditions:challenges and opportunities[J]. Angewan- dte Chemie:International Edition, 2020,59.Doi: 10.1002/ange.201909339. |
| [1] | SHI Wangfang, ZHANG Yongsheng. Study on NO x degradation performance of concrete-based non-metallic boron doped nitrogen-rich carbon nitride [J]. Inorganic Chemicals Industry, 2025, 57(3): 116-123. |
| [2] | LI Zihan, ZHANG Jiaqi, LI Shizhuo, LI Xinyu, LIU Shaozhuo, WANG Yihao, HAO Yucui, LIU Jian, LI Yanhua. Study on synthesis and catalytic mechanism of CdS/g-C3N4 composite photocatalyst [J]. Inorganic Chemicals Industry, 2025, 57(3): 124-132. |
| [3] | LUO Chengling, FAN Xiaofan. Research progress of microstructure-regulated catalysts for urea oxidation reactions [J]. Inorganic Chemicals Industry, 2025, 57(2): 26-35. |
| [4] | SUN Qinghao, LI Keyan, GUO Xinwen. Study on photocatalytic benzyl alcohol oxidation coupled with hydrogen production over Pd/ZnIn2S4 nanosheets [J]. Inorganic Chemicals Industry, 2025, 57(1): 113-119. |
| [5] | LIU Guangming. Study on photocatalytic and mechanical properties of C3N5/NH2-MIL-125(Ti) modified concrete mortar [J]. Inorganic Chemicals Industry, 2025, 57(1): 120-128. |
| [6] | ZHANG Guoqiang, RONG Xilin, XIAO Zhenfang, XUE Ziran, CHENG Hao, FENG Jun, LIU Quan, LU Yao, HUANG Wenyi. Study on preparation and photocatalytic properties of bagasse carbon aerogels loaded with zinc oxide nanoparticles [J]. Inorganic Chemicals Industry, 2024, 56(8): 131-138. |
| [7] | WANG Ting, ZHANG Wenwen, MAO Qing, LÜ Li, LIU Changzhen. Research progress of catalytic system and materials for electrocatalytic reduction of carbon dioxide to ethanol [J]. Inorganic Chemicals Industry, 2024, 56(7): 1-10. |
| [8] | WANG Yawen, WANG Fangfang, GENG Siyu, JU Jia, CHEN Lei, CHEN Changdong. Study on preparation and photocatalytic performance of SrTiO3-SrWO4 [J]. Inorganic Chemicals Industry, 2024, 56(7): 143-149. |
| [9] | LIU Min, HUANG Xiu, ZHANG Liyuan. Research progress of S-type heterojunction photocatalysts [J]. Inorganic Chemicals Industry, 2024, 56(7): 18-27. |
| [10] | LI Jiangpeng, ZHANG Huibin. Synergistic degradation of methylene blue by photo-Fenton and photocatalytic with 3D porous LaFeO3/CeO2/SrTiO3 [J]. Inorganic Chemicals Industry, 2024, 56(5): 141-148. |
| [11] | XIE Jiang, GUO Ge, QIU Jie. Treatment of methylene blue simulated wastewater by supported activated carbon particle with three-dimensional electrode method [J]. Inorganic Chemicals Industry, 2024, 56(5): 78-86. |
| [12] | TANG Bei. Preparation of ZnO/g-C3N4 heterojunction photocatalytic material and its degradation of pyridine [J]. Inorganic Chemicals Industry, 2024, 56(4): 133-142. |
| [13] | ZHOU Xuan, LI Mengrui, CHEN Yichen, FAN Huiqiang, WANG Bin, YUAN Gang. Research progress of nickel-based phosphide composites in improving of catalytic water electrolysis for hydrogen evolution performance [J]. Inorganic Chemicals Industry, 2024, 56(4): 8-15. |
| [14] | HUANG Jianan, LU Xiaoyu, WANG Mitang. Effect of Ba-La co-doping on degradation of methylene blue dye by TaON [J]. Inorganic Chemicals Industry, 2024, 56(2): 146-151. |
| [15] | CHEN Xingliang, FAN Wenjuan, CHANG Hui, HUANG Haiping, JIANG Zhiqiang. Study on collaborative strategy between Fe3+ and Ni-based metal-organic frameworks for boosting electrocatalytic oxygen evolution [J]. Inorganic Chemicals Industry, 2024, 56(2): 152-158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||