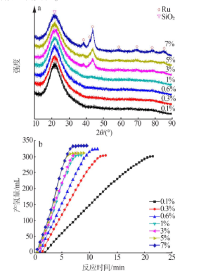
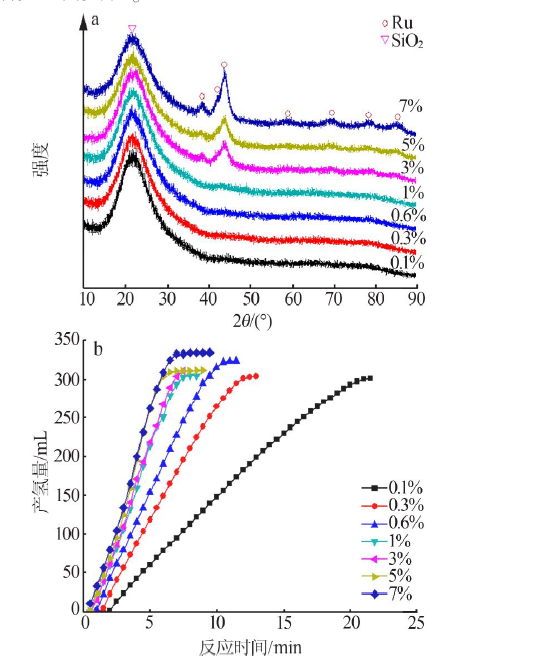
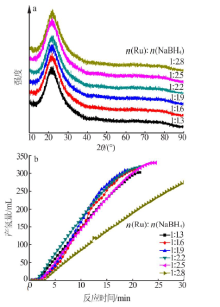
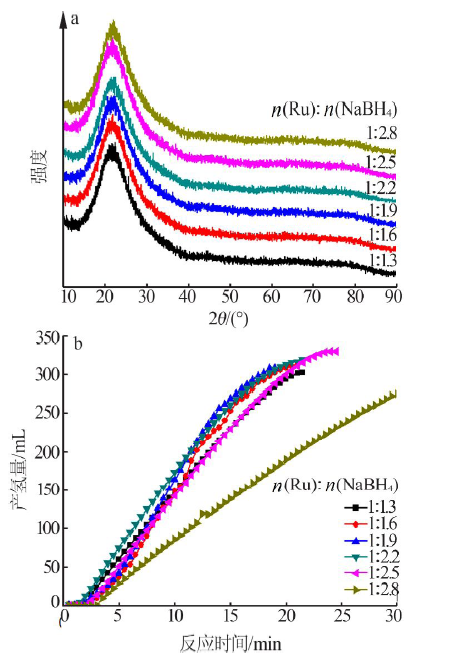
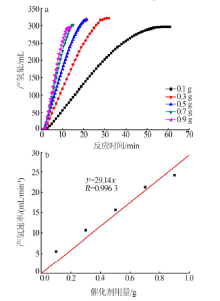
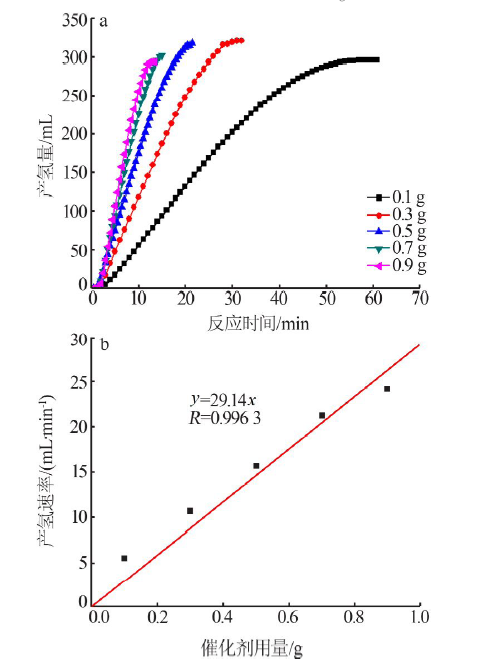
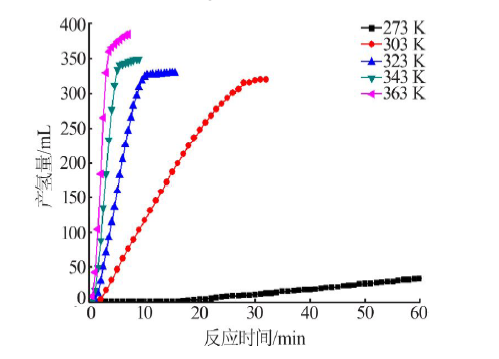
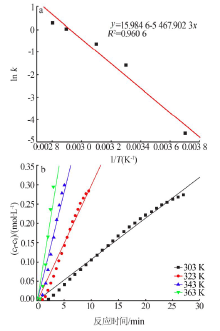
| [1] |
孙海杰, 陈凌霞, 张玉凤 , 等. 钴-硼/二氧化锆催化剂催化硼氢化钠水解制氢研究[J]. 无机盐工业, 2019,51(3):72-76.
|
| [2] |
孙海杰, 陈凌霞, 黄振旭 , 等. 第四周期过渡金属催化硼氢化钠分解制氢研究[J]. 无机盐工业, 2017,49(5):14-17.
|
| [3] |
张磊, 涂倩, 陈学年 , 等. 氨硼烷释氢纳米金属催化剂的研究[J]. 化学进展, 2014,26(5):749-755.
|
| [4] |
杨兰, 罗威, 程功臻 . 氨硼烷水解制氢的研究进展[J]. 大学化学, 2014,26(6):1-10.
|
| [5] |
李慧珍, 王芃远, 陈学年 . 氨硼烷:一种高性能化学储氢材料[J]. 科学通报, 2014,59(19):1823-1837.
|
| [6] |
马建丽, 张晓霞, 曹海燕 , 等. 氨硼烷热分解放氢的研究进展[J]. 化工新型材料, 2016,44(5):34-36.
|
| [7] |
杨晓婧, 尚伟, 李兰兰 , 等. 金属催化氨硼烷制氢研究进展[J]. 电源技术, 2014,38(7):1387-1389.
|
| [8] |
桑婉璐, 李兰兰, 高若源 , 等. 氨硼烷水解制氢催化剂载体的研究进展[J]. 材料导报, 2017,31(9):27-33.
|
| [9] |
徐凤勤, 胡小飞, 程方益 , 等. 多孔碳负载镍纳米颗粒的制备及催化氨硼烷水解制氢[J]. 无机化学学报, 2015,31(1):103-108.
|
| [10] |
占林军, 孙晓燕, 周瑛 , 等. 铈基复合氧化物催化剂在SiO2表面的失活机制[J]. 物理化学学报, 2017,33(7):1474-1482.
|
| [11] |
孙海杰, 陈凌霞, 黄振旭 , 等. 还原介质和还原温度对Ru-Zn催化苯选择加氢制环己烯性能的影响[J]. 化工进展, 2017,36(8):2962-2970.
|
| [12] |
Hu S C, Chen Y W . Partial hydrogenation of benzene to cyclohexene on ruthenium catalysts supported on La2O3-ZnO binary oxides[J]. Ind. Eng.Chem.Res., 1997,36:5153-5159.
|
| [13] |
王海霞, 周丽敏, 陶占良 , 等. Cu@Co纳米颗粒合成及催化氨硼烷水解放氢性能[J]. 功能材料与器件学报, 2015,21(4):7-12.
|
| [14] |
Giovanni P R, Umit B D, Philippe M , et al. Facile synjournal by polyol method of a ruthenium catalyst supported on γ-Al2O3 for hydrolytic dehydrogenation of ammonia borane[J]. Catal.Today, 2011,170(1):85-92.
|
| [15] |
Basu S, Brockman A, Gagare P , et al. Chemical kinetics of Rucatalyzed ammonia borane hydrolysis[J]. J Power Sources, 2009,188:238-243.
|
| [16] |
Can H, Metin Ö . A facile synjournal of early monodisperse ruthenium nanoparticles and their catalysis in the hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage[J]. Appl.Catal.B:Environmental, 2012,125(3):304-310.
|
| [17] |
Serdar A, Pelin E, Saim Ö . Hydroxyapatite supported ruthenium(0) nanoparticles catalyst in hydrolytic dehydrogenation of ammonia borane:Insight to the nanoparticles formation and hydrogen evolution kinetics[J]. Appl.Catal.B:Environmental, 2013,142/143:187-195.
|
| [18] |
陈健民, 卢章辉, 熊丽华 . Ru/Ce(OH)CO3纳米复合材料催化氨硼烷水解产氢[J]. 无机化学学报, 2016,32(10):1816-1824.
|
 ),Chen Lingxia(
),Chen Lingxia( ),Li Congcong,Mei Yangyang
),Li Congcong,Mei Yangyang