Inorganic Chemicals Industry ›› 2026, Vol. 58 ›› Issue (1): 74-83.doi: 10.19964/j.issn.1006-4990.2025-0079
• Environment·Health·Safety • Previous Articles Next Articles
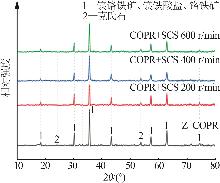
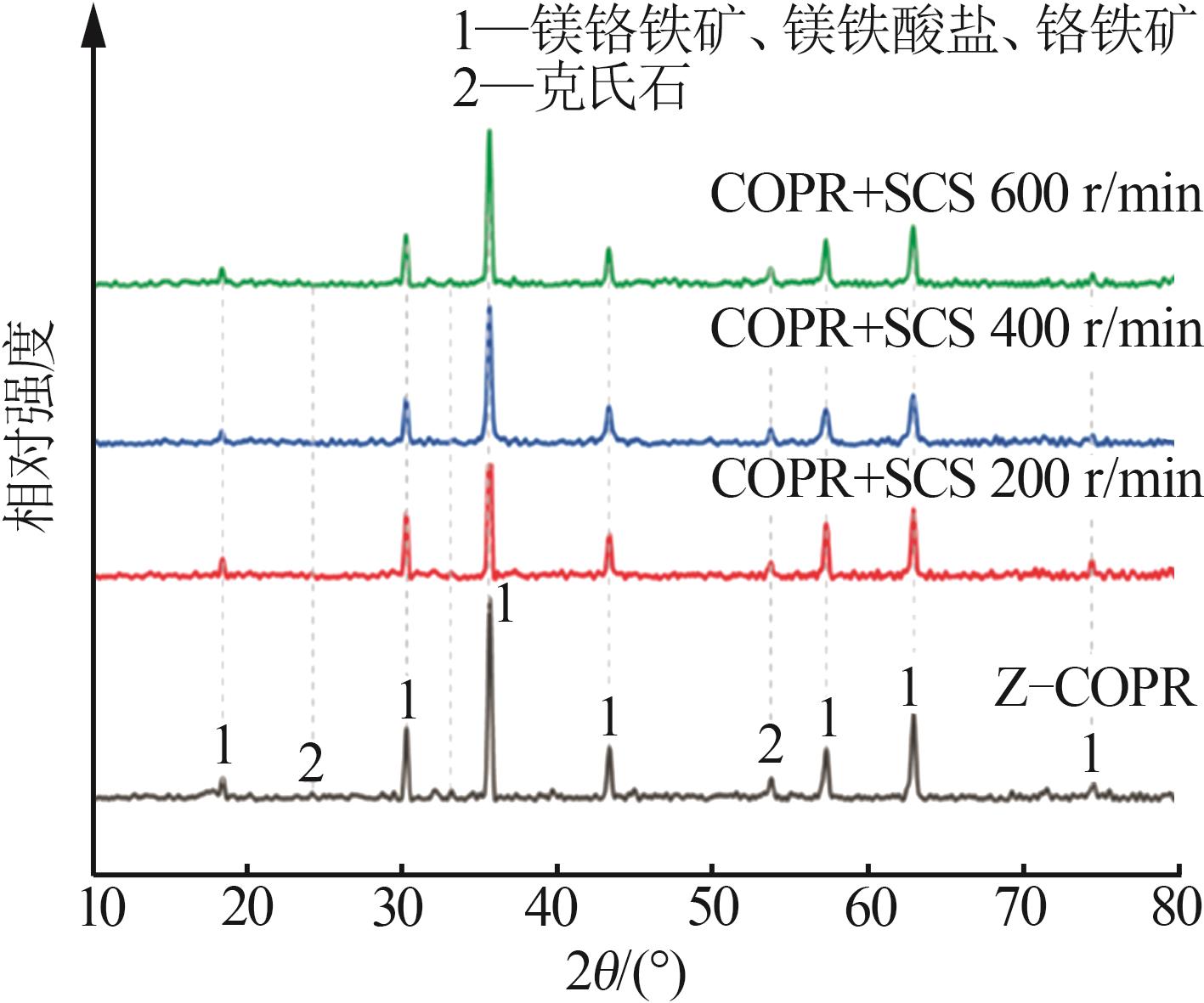
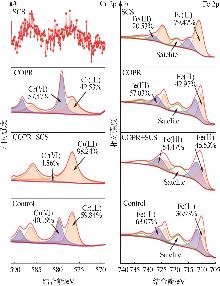
Study on reductive detoxification of calcium⁃free roasted chromite ore processing residue via mechanochemical activation with secondary copper slag
ZHAO Zhiying1,2( ), ZHAO Xiaolong1,2, CHEN Xiaohong1,2, DU Ying1,2, DU Dongyun1,2(
), ZHAO Xiaolong1,2, CHEN Xiaohong1,2, DU Ying1,2, DU Dongyun1,2( )
)
- 1. Hubei Engineering Research Center for Heavy Metal Pollution Prevention and Control,School of Resources and Environment,Central South University for Nationalities,Wuhan 430074,China
2. Key Laboratory of Catalytic Transformation and Energy Materials Chemistry,Ministry of Education,Central South University for Nationalities,Wuhan 430074,China
-
Received:2025-02-19Online:2026-01-10Published:2025-06-30 -
Contact:DU Dongyun E-mail:zhaozhiying8710@163.com;dydu666@mail.scuec.edu.cn
CLC Number:
Cite this article
ZHAO Zhiying, ZHAO Xiaolong, CHEN Xiaohong, DU Ying, DU Dongyun. Study on reductive detoxification of calcium⁃free roasted chromite ore processing residue via mechanochemical activation with secondary copper slag[J]. Inorganic Chemicals Industry, 2026, 58(1): 74-83.
share this article
Table 2
Response surface regression model ANOVA results"
| 来源 | 方差平方和 | Df | 均方差 | F值 | P值 | 显著性 |
|---|---|---|---|---|---|---|
| 模型 | 53 772.84 | 20 | 2 688.64 | 115.19 | <0.000 1 | 显著 |
| A | 15 886.08 | 1 | 15 886.08 | 680.60 | <0.000 1 | 显著 |
| B | 16 674.10 | 1 | 16 674.10 | 714.36 | <0.000 1 | 显著 |
| C | 4 812.68 | 1 | 4 812.68 | 206.19 | <0.000 1 | 显著 |
| D | 2 744.74 | 1 | 2 744.74 | 117.59 | <0.000 1 | 显著 |
| E | 11.14 | 1 | 11.14 | 0.477 2 | 0.496 0 | 不显著 |
| AB | 909.54 | 1 | 909.54 | 38.97 | <0.000 1 | 显著 |
| AC | 1 692.09 | 1 | 1 692.09 | 72.49 | <0.000 1 | 显著 |
| AD | 539.91 | 1 | 539.91 | 23.13 | <0.000 1 | 显著 |
| AE | 36.37 | 1 | 36.37 | 1.56 | 0.223 5 | 不显著 |
| BC | 1 389.09 | 1 | 1 389.09 | 59.51 | <0.000 1 | 显著 |
| BD | 61.73 | 1 | 61.73 | 2.64 | 0.116 4 | 不显著 |
| BE | 57.52 | 1 | 57.52 | 2.46 | 0.129 0 | 不显著 |
| CD | 754.33 | 1 | 754.33 | 32.32 | <0.000 1 | 显著 |
| CE | 31.77 | 1 | 31.77 | 1.36 | 0.254 4 | 不显著 |
| DE | 42.15 | 1 | 42.15 | 1.81 | 0.191 1 | 不显著 |
| 残差 | 583.53 | 25 | 23.34 | |||
| 失拟项 | 496.63 | 20 | 24.83 | 1.43 | 0.370 1 | 不显著 |
| 纯误差 | 86.91 | 5 | 17.38 | |||
| 总离差 | 54 356.37 | 45 | ||||
| R2=0.961 2 |
| [1] | 胡念,柯军,邱远卓,等.铬渣无害化处理研究进展[J].应用化工,2024,53(10):2458-2463. |
| HU Nian, KE Jun, QIU Yuanzhuo,et al.Research progress in harmless treatment of chromium slag[J].Applied Chemical Industry,2024,53(10):2458-2463. | |
| [2] | WANG Wentao, CHEN Chunmei, HUANG Xiaopeng,et al.Chromium(Ⅵ) adsorption and reduction in soils under anoxic conditions:The relative roles of iron(oxyhr)oxides,iron(Ⅱ),organic matters,and microbes[J].Environmental Science & Technology,2024,58(41):18391-18403. |
| [3] | 蹇成宗,蒋子文,全学军,等.铬铁矿无钙焙烧渣中铬的绿色提取[J].无机盐工业,2024,56(7):118-125. |
| JIAN Chengzong, JIANG Ziwen, QUAN Xuejun,et al.Eco⁃friendly extraction of chromium from calcium⁃free roasting slag of chromite ore[J].Inorganic Chemicals Industry,2024,56(7):118-125. | |
| [4] | 蒋子文,全学军,李纲,等.铬渣资源化利用研究进展[J].无机盐工业,2023,55(2):26-35. |
| JIANG Ziwen, QUAN Xuejun, LI Gang,et al.Research progress on resource utilization of chromium slag[J].Inorganic Chemicals Industry,2023,55(2):26-35. | |
| [5] | 谢雅骐,汪岚玢,杜亚光.软锰矿协同铬渣水热回收铬和锰[J].中南民族大学学报(自然科学版),2024,43(1):16-23. |
| XIE Yaqi, WANG Lanbin, DU Yaguang.Hydrothermal recovery of chromium and manganese using pyrolusite with chromite ore processing residue[J].Journal of South⁃Central Minzu University (Natural Science Edition),2024,43(1):16-23. | |
| [6] | XU Ju, LIU Mengke, MA Guojun,et al.Valuable recovery technology and resource utilization of chromium⁃containing metallurgical dust and slag:A review[J].Metals,2023,13(10):1768. |
| [7] | 胡继娟,王小治,侯建华.碳基材料负载纳米零价铁对铬废水的处理研究进展[J].现代化工,2025,45(2):63-67. |
| HU Jijuan, WANG Xiaozhi, HOU Jianhua.Research progress in treatment of chromium⁃containing wastewater by carbon⁃based materials loaded with nanoscale zerovalent iron[J].Modern Chemical Industry,2025,45(2):63-67. | |
| [8] | XU Rong, WANG Yanan, LI Shupeng,et al.Effective Cr(Ⅵ) reduction and immobilization in chromite ore processing residue (COPR) contaminated soils by ferrous sulfate and digestate:A comparative investigation with typical reducing agents[J].Ecotoxicology and Environmental Safety,2023,265:115522. |
| [9] | 文惠子,习路遥,何淑玉,等.Na2CO3强化食品添加剂废水水热湿法还原解毒铬渣的研究[J].无机盐工业,2025,57(1):83- 89. |
| WEN Huizi, XI Luyao, HE Shuyu,et al.Research on Na2CO3 enhanced food additive wastewater hydrothermal wet reduction and detoxification of chromite ore processing residue[J].Inorganic Che⁃Industry micals,2025,57(1):83-89. | |
| [10] | LAN Yingying, ZHANG Lijuan, LI Xiaoqin,et al.Efficient immobilization and utilization of chromite ore processing residue via hydrothermally constructing spinel phase Fe2+(Cr3+ x,Fe3+2- x )O4 and its magnetic separation[J].Science of the Total Environment,2022,813:152637. |
| [11] | LIU Zhangbin, ZHENG Jiayi, LIU Weizhen,et al.Identification of the key host phases of Cr in fresh chromite ore processing residue(COPR)[J].Science of the Total Environment,2020,703:135075. |
| [12] | XIE Junlin, WEI Kun, LIU Xiaoqing,et al.Effect of carbon type on the detoxification mechanism of hexavalent chromium[Cr(Ⅵ)] by carbothermal reduction[J].Journal of Environmental Chemical Engineering,2022,10(4):108091. |
| [13] | DU Yaguang, CHRYSOCHOOU M.Microstructural analyses of Cr(Ⅵ) speciation in chromite ore processing residue from the soda ash process[J].Journal of Hazardous Materials,2020,393:122385. |
| [14] | TIAN Hong, WANG Lanbin, XU Yangming,et al.Efficient reduction of Cr(Ⅵ) and recovery of Fe from chromite ore processing residue by waste biomass[J].Environmental Technology & Innovation,2023,30:103046. |
| [15] | JIN Hang, YUAN Bihe, XU Xichen,et al.Detoxification of chromium slag using corn stalk for the hierarchical preparation of ultrafine aluminum hydroxide and iron hydroxide[J].Powder Technology,2024,437:119575. |
| [16] | CHEN Jie, ZHANG Xingran, WANG Yan,et al.Research progress on hazardous chromite ore processing residue treatment and utilization:A critical review[J].Chemical Engineering Communications,2024,211(9):1445-1468. |
| [17] | 曾一钧,蒋子文,蹇成宗,等.铬铁矿无钙焙烧渣中铬的深度提取研究[J].无机盐工业,2025,57(1):90-96. |
| ZENG Yijun, JIANG Ziwen, JIAN Chengzong,et al.Study on deep extraction of chromium from calcium⁃free roasting slag of chromite ore[J].Inorganic Chemicals Industry,2025,57(1):90-96. | |
| [18] | 韦应,蒋章豪,全学军,等.铬渣生物质还原制备臭氧催化剂及其性能[J].中国有色金属学报,2024,34(12):4126-4137. |
| WEI Ying, JIANG Zhanghao, QUAN Xuejun,et al.Preparation of ozone catalyst by thermal reduction of chromium residue with biomass and its performance[J].The Chinese Journal of Nonferrous Metals,2024,34(12):4126-4137. | |
| [19] | TIAN Cheng, LU Cunfang, YANG Jun,et al.An effective strate⁃gy for high⁃value utilization of chromite ore processing residue(COPR) through reductive alkali roasting and physical separation[J].Process Safety and Environmental Protection,2025,193:874-885. |
| [20] | 刘雨涵,高盼.使用机械化学生成的钙基重格氏试剂(R-CaX)对有机卤化物进行直接硼化[J].化学学报,2024,82(11):1114-1119. |
| LIU Yuhan, GAO Pan.Direct borylation of organohalides using mechanochemically generated calcium⁃based heavy Grignard reagents(R-CaX)[J].Acta Chimica Sinica,2024,82(11):1114-1119. | |
| [21] | 张将,贺会军,付东兴,等.机械化学法制备Cu/Fe复合粉末及性能研究[J].稀有金属,2024,48(10):1417-1425. |
| ZHANG Jiang, HE Huijun, FU Dongxing,et al.Mechanochemical preparation and properties of Cu/Fe composite powder[J].Chinese Journal of Rare Metals,2024,48(10):1417-1425. | |
| [22] | ZHENG Xingfu, CAO Siting, NIE Zhenyuan,et al.Impact of mechanical activation on bioleaching of pyrite:A DFT study[J].Minerals Engineering,2020,148:106209. |
| [23] | SUN Yan, DU Yaguang, LAN Jirong,et al.A new method(ball milling and sodium sulfide) for mechanochemical treatment of soda ash chromite ore processing residue[J].Journal of Hazardous Materials,2021,415:125601. |
| [24] | 高恩霞,姜涛,崔石岩,等.铜渣与高炉灰共还原-磁选工艺回收铁试验研究[J].中国有色冶金,2022,51(6):118-124. |
| GAO Enxia, JIANG Tao, CUI Shiyan,et al.Optimization of iron recovery from copper slag and blast furnace dust by co⁃reduction followed by magnetic separation[J].China Nonferrous Metallurgy,2022,51(6):118-124. | |
| [25] | 薛超龙,李慧,梁精龙,等.铜渣资源回收的研究现状及展望[J].中国冶金,2022,32(2):108-114. |
| XUE Chaolong, LI Hui, LIANG Jinglong,et al.Research status and prospects of copper slag resource recovery[J].China Metallurgy,2022,32(2):108-114. | |
| [26] | JAROŠÍKOVÁ A, ETTLER V, MIHALJEVIČ M,et al.Transformation of arsenic⁃rich copper smelter flue dust in contrasting soils:A 2-year field experiment[J].Environmental Pollution,2018,237:83-92. |
| [27] | 李金惠,张上,孙乾予.我国工业固体废物处理利用产业状况分析与展望[J].环境保护,2021,49(2):14-18. |
| LI Jinhui, ZHANG Shang, SUN Qianyu.Analysis and prospect of industrial solid waste treatment and utilization industry in China[J].Environmental Protection,2021,49(2):14-18. | |
| [28] | LIU Ranran, HOU Lanlan, YUE Guichu,et al.Progress of fabrication and applications of electrospun hierarchically porous nanofibers[J].Advanced Fiber Materials,2022,4(4):604-630. |
| [29] | CHEN Xiaohong, KE Xuan, ZHAO Zhiying,et al.Copper slag⁃aided mechanochemical detoxification soda ash chromite ore processing residue and long⁃term stabilization:Efficient reduction and reoccurrence inhibition of Cr(Ⅵ)[J].Journal of Cleaner Production,2025,488:144674. |
| [30] | JIANG Bo, GONG Yifan, GAO Jianan,et al.The reduction of Cr(Ⅵ) to Cr(Ⅲ) mediated by environmentally relevant carboxylic acids:State⁃of⁃the⁃art and perspectives[J].Journal of Hazardous Materials,2019,365:205-226. |
| [31] | 顾屹仕,沈中杰,袁鼎轩,等.颗粒尺度对CaO颗粒团簇水合反应的传质影响[J].华东理工大学学报(自然科学版),2025,51(1):1-9. |
| GU Yishi, SHEN Zhongjie, YUAN Dingxuan,et al.Effect of particle size on mass transfer in hydration reaction of CaO particle clusters[J].Journal of East China University of Science and Technology (Natural Science Edition),2025,51(1):1-9. | |
| [32] | LI Xian, GRAHAM N J D, DENG Wensheng,et al.Structural variation of precipitates formed by Fe(Ⅱ) oxidation and impact on the retention of phosphate[J].Environmental Science & Technology,2022,56(7):4345-4355. |
| [33] | WANG Lanbin, SUN Yan, DU Yaguang,et al.Treatment of soda ash chromite ore processing residue by Waste⁃Molasses⁃Based Ball Milling:A new strategy for disposal of waste with waste[J].Journal of Cleaner Production,2022,374:133981. |
| [34] | LAPSHIN O V, BOLDYREVA E V, BOLDYREV V V.Role of mixing and milling in mechanochemical synthesis(review)[J].Russian Journal of Inorganic Chemistry,2021,66(3):433-453. |
| [35] | 安邦,徐明聪,马春慧,等.纤维素纳米晶体手性复合材料:结构色的调控与应用[J].高分子学报,2022,53(3):211- 226. |
| AN Bang, XU Mingcong, MA Chunhui,et al.Tuning and application of structural color of cellulose nanocrystals chiral composite materials[J].Acta Polymerica Sinica,2022,53(3):211-226. | |
| [36] | HUA Tianci, LI Yanzhang, HOU Bingxu,et al.Chromium immobilization as Cr⁃spinel by regulation of Fe(Ⅱ) and Fe(Ⅲ) concentrations[J].Minerals,2024,14(10):1024. |
| [1] | SHEN Mengmeng. Study on preparation of g-C3N4/polyaniline composites and their application in photocatalytic reduction of CO2 [J]. Inorganic Chemicals Industry, 2025, 57(8): 123-130. |
| [2] | ZHAO Yanyan, TIAN Zhuangzhuang. Study on preparation and visible light degradation performance of g-C3N4 loaded nano-Ag composites [J]. Inorganic Chemicals Industry, 2025, 57(6): 114-122. |
| [3] | SHEN Xiaoqian, ZHOU Fei, LIU Wanchen, XU Lu, WU Junshu. Study on synthesis of FeS modified calcium silicate hydrate composites and their total Cr removal performance [J]. Inorganic Chemicals Industry, 2025, 57(2): 57-67. |
| [4] | MA Caimei, JIN Hua, ZHOU Wen. Study on preparation of CuO-PMA heterogeneous nanosheets and their photocatalytic CO2 reduction [J]. Inorganic Chemicals Industry, 2025, 57(11): 130-136. |
| [5] | WEN Huizi, XI Luyao, HE Shuyu, TAN Shanyi, ZHANG Liwen, CHEN Shaohua, DU Yaguang. Research on Na2CO3 enhanced food additive wastewater hydrothermal wet reduction and detoxification of chromite ore processing residue [J]. Inorganic Chemicals Industry, 2025, 57(1): 83-89. |
| [6] | SHI Mengke, FAN Zhaoya, YUE Feng, ZHANG Shuo, MENG Yang, ZHANG Hongzhong. Study on electro-assisted photocatalytic high selective conversion of CO2 in air [J]. Inorganic Chemicals Industry, 2024, 56(9): 154-163. |
| [7] | WANG Ting, ZHANG Wenwen, MAO Qing, LÜ Li, LIU Changzhen. Research progress of catalytic system and materials for electrocatalytic reduction of carbon dioxide to ethanol [J]. Inorganic Chemicals Industry, 2024, 56(7): 1-10. |
| [8] | JIAN Chengzong, JIANG Ziwen, QUAN Xuejun, LI Gang. Eco⁃friendly extraction of chromium from calcium⁃free roasting slag of chromite ore [J]. Inorganic Chemicals Industry, 2024, 56(7): 118-125. |
| [9] | LIU Min, HUANG Xiu, ZHANG Liyuan. Research progress of S-type heterojunction photocatalysts [J]. Inorganic Chemicals Industry, 2024, 56(7): 18-27. |
| [10] | LAI Huilong, YU fei, YANG Dongxia, MA Jiangli, YIN Xuemei, CHANG Shiying. Research on NH3-SCR performance and ammonia storage characteristics based on different Cu-CHA catalyst schemes [J]. Inorganic Chemicals Industry, 2024, 56(12): 159-166. |
| [11] | ZHAO Yan, HAO Xuewei, SHI Hainan, LI Jiahui, LI Keyan, GUO Xinwen. Study on photocatalytic CO2 reduction performance of Cu-doped TiO2/PCN heterojunction [J]. Inorganic Chemicals Industry, 2023, 55(8): 21-27. |
| [12] | SONG Zhijia, WANG Suisui, KUANG Qin. Hollow Cu-doped TiO2 for enhancing photocatalytic CO2 reduction performance [J]. Inorganic Chemicals Industry, 2023, 55(8): 45-50. |
| [13] | LI Peng, YANG Siyuan, LUO Shizhong, DU Peining, TAN Renjun, JING Fangli. Neodymium-modified iron-based catalysts for nitrogen oxides removal [J]. Inorganic Chemicals Industry, 2023, 55(3): 140-146. |
| [14] | PENG Shuang, CHEN Chaoyi, WANG Shiyu, BAI Yang, LI Tianpei, GU Wei. Study on preparation of titanium carbide derived carbon by electrolysis-etching in CaCl2 molten salt [J]. Inorganic Chemicals Industry, 2023, 55(3): 78-83. |
| [15] | WANG Xuemeng, AN Yan, LIU Hai, TIAN Mengkui. Carbon footprint analysis of yellow phosphorus products based on LCA [J]. Inorganic Chemicals Industry, 2023, 55(12): 36-42. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||