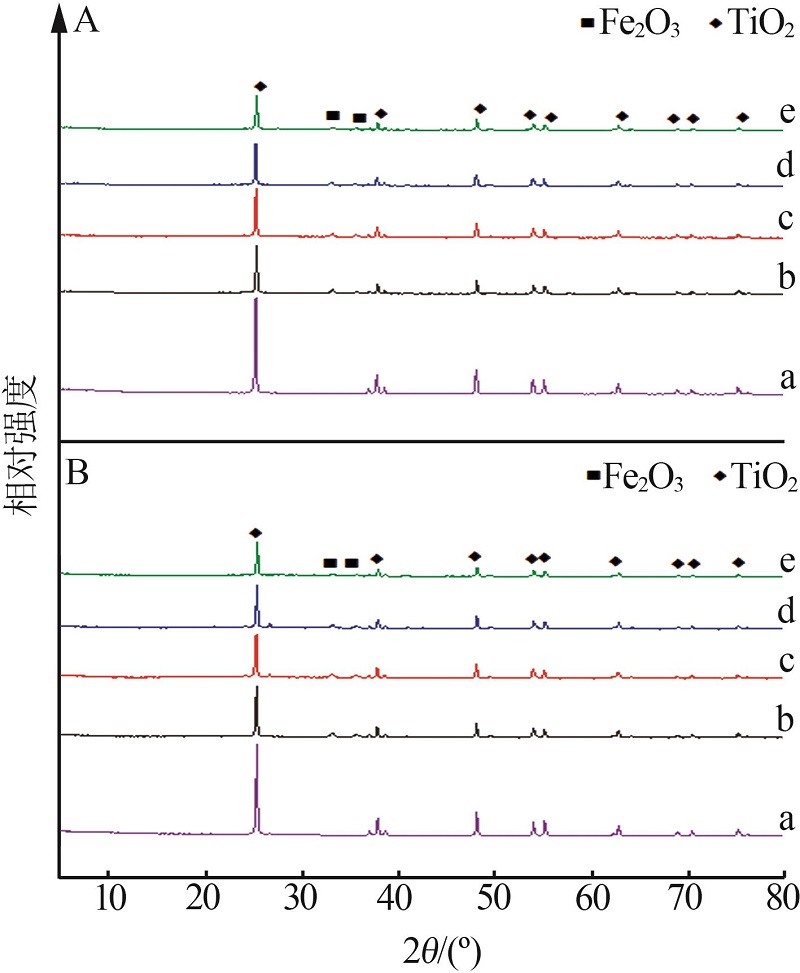
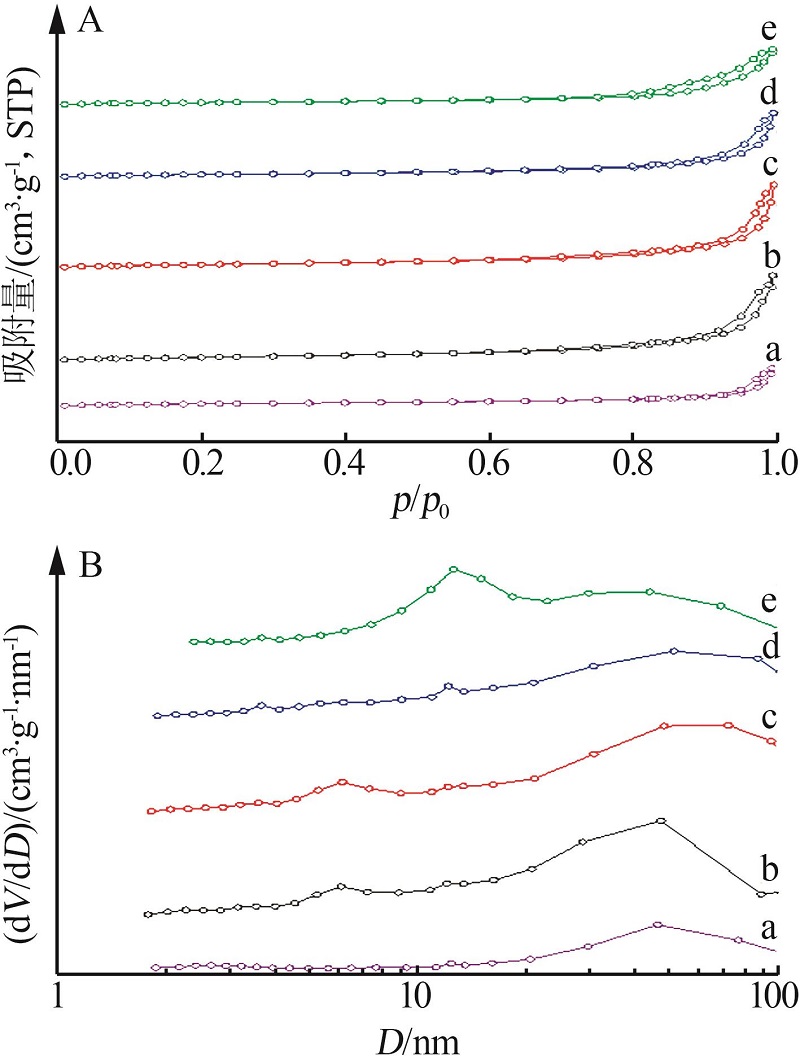
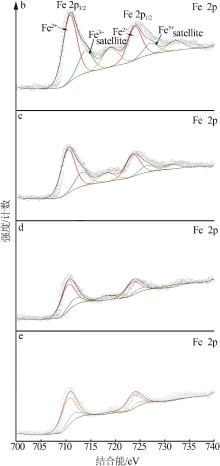
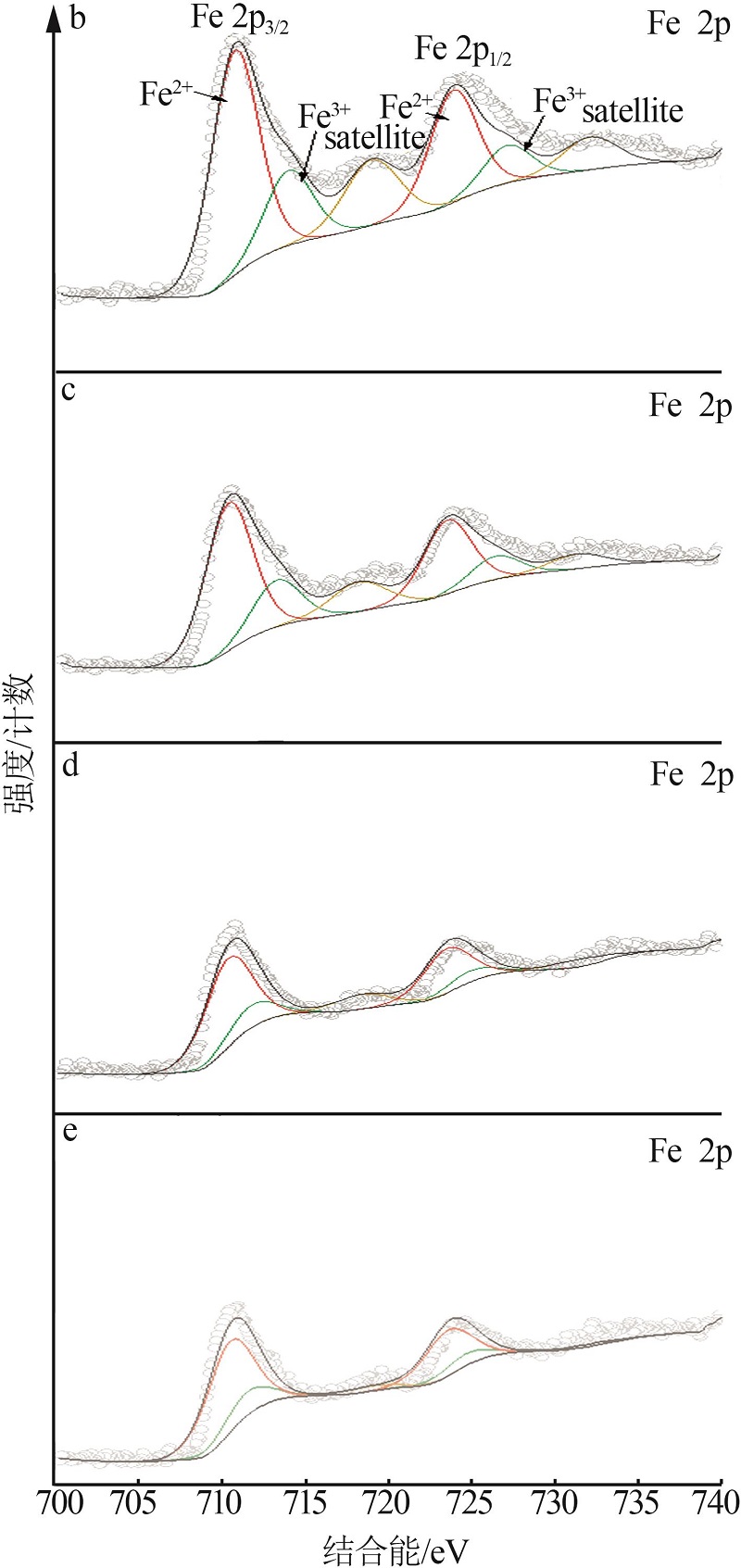
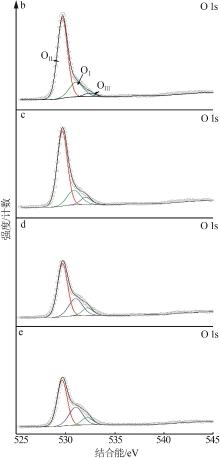
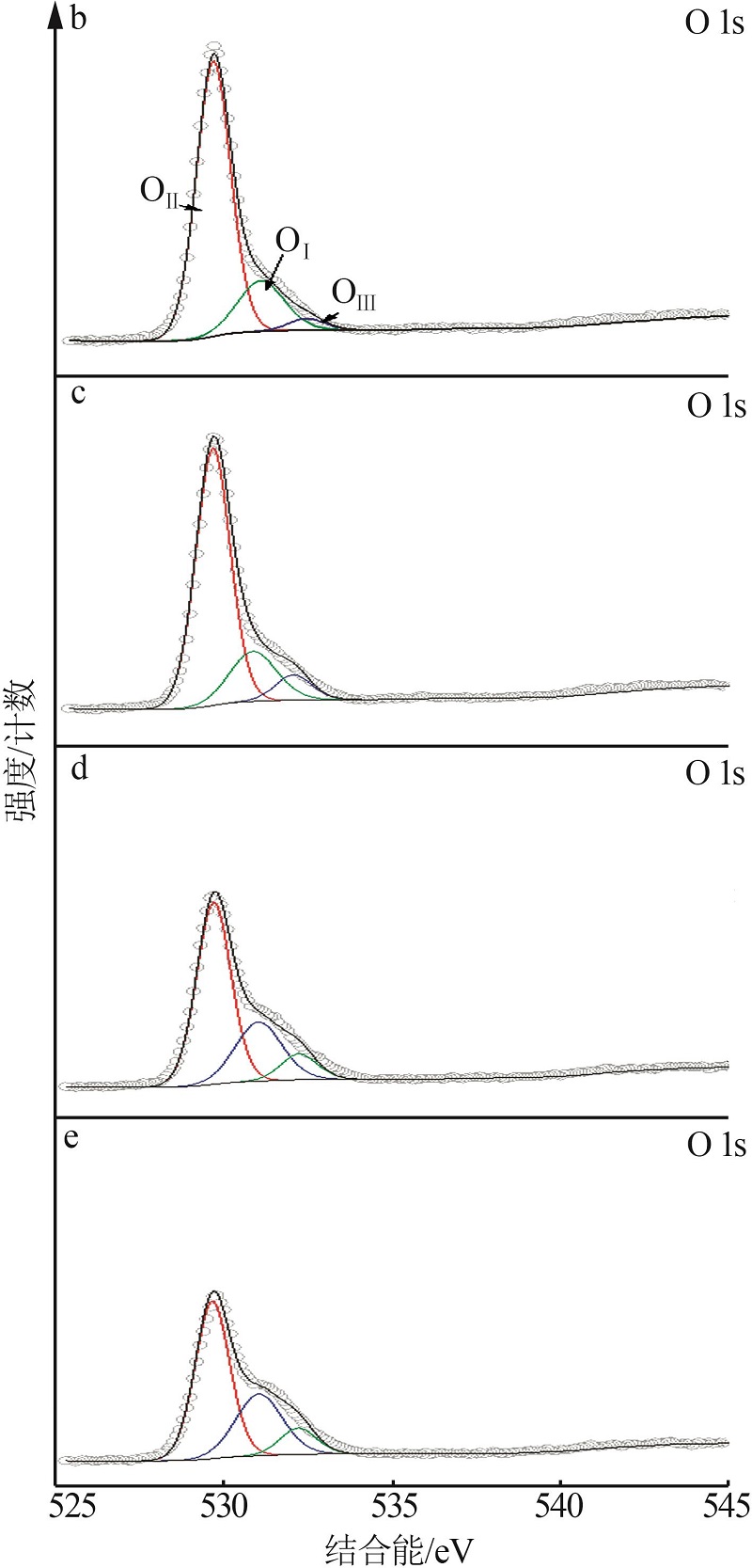
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (3): 140-146.doi: 10.19964/j.issn.1006-4990.2022-0366
• Catalytic Materials • Previous Articles
Neodymium-modified iron-based catalysts for nitrogen oxides removal
LI Peng1( ), YANG Siyuan1, LUO Shizhong2, DU Peining2, TAN Renjun2, JING Fangli3
), YANG Siyuan1, LUO Shizhong2, DU Peining2, TAN Renjun2, JING Fangli3
- 1. Institute of Petroleum Engineering,Northwest Oilfield Branch,SINOPEC,Urumqi 830011,China
2. School of Chemical Engineering,Sichuan University,Chengdu 610065,China
3. College of Chemistry and Chemical;Engineering,Southwest Petroleum University,Chengdu 610500,China