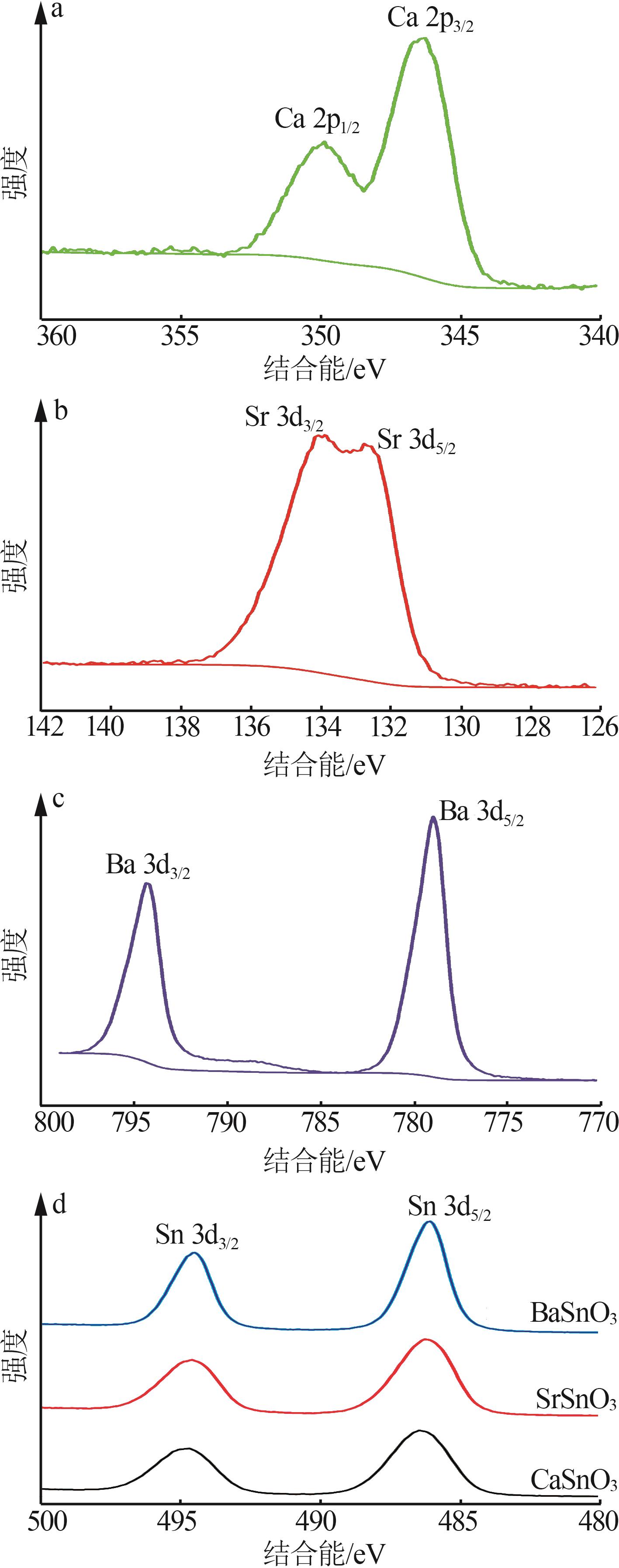
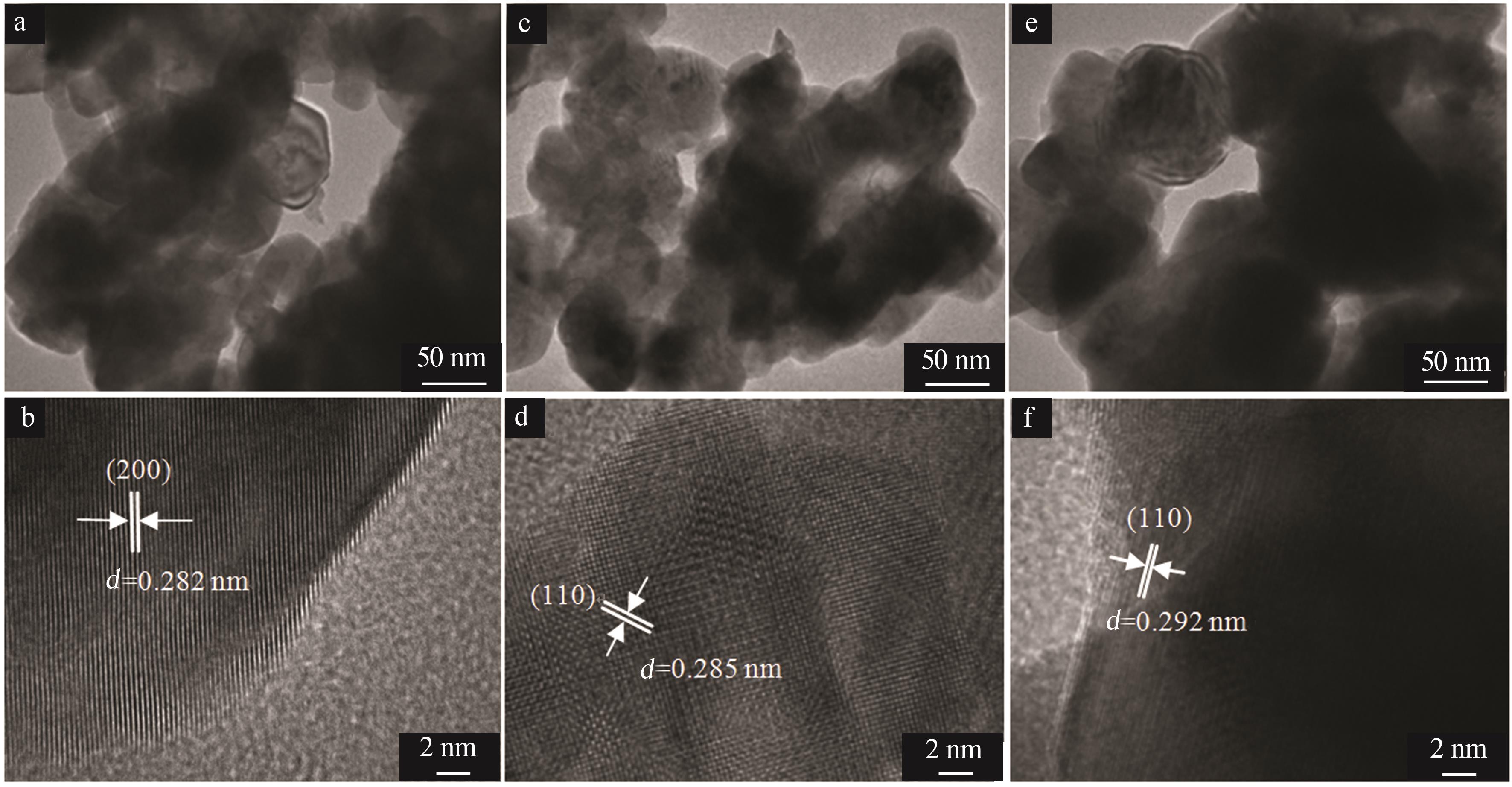
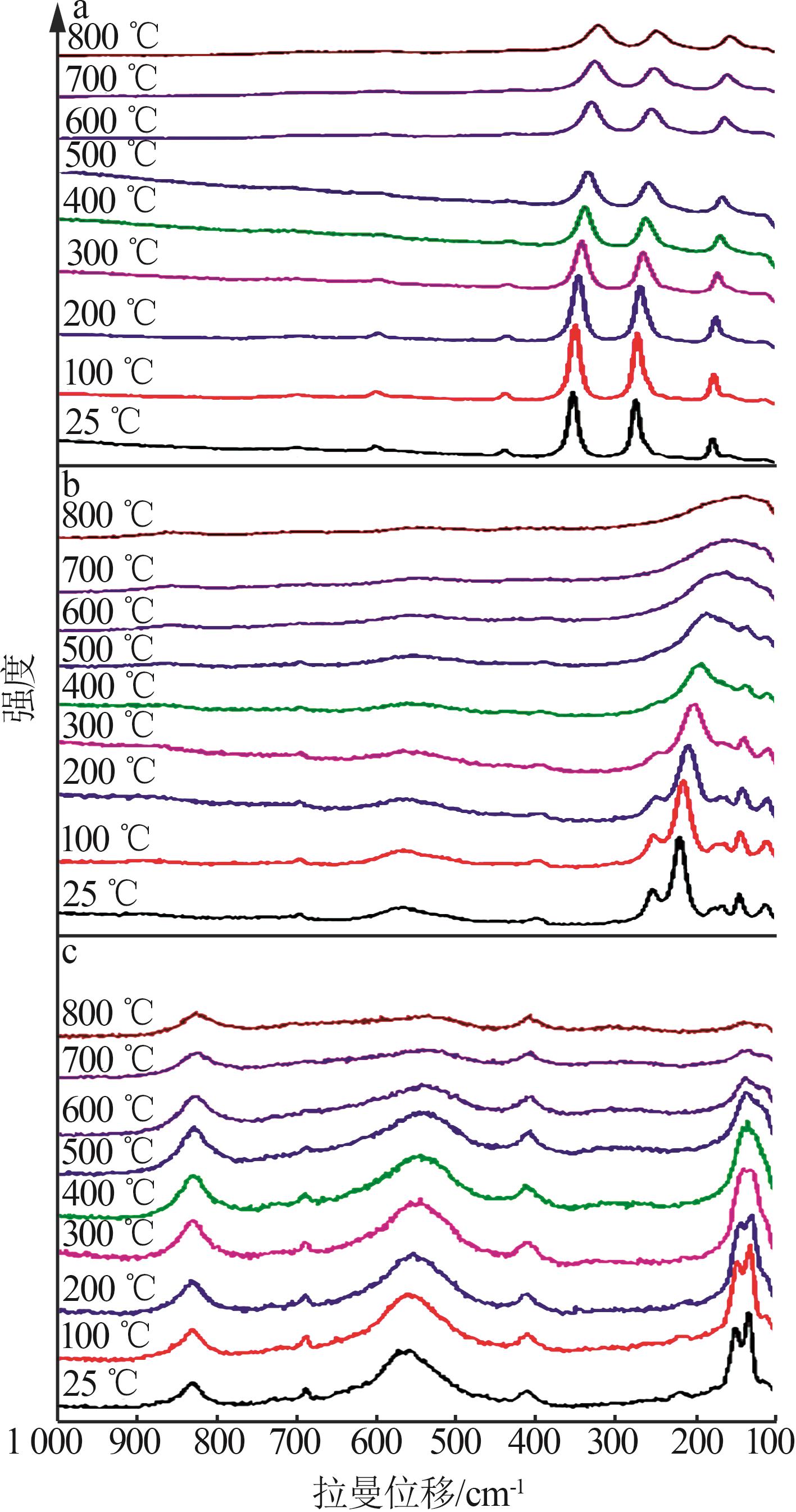
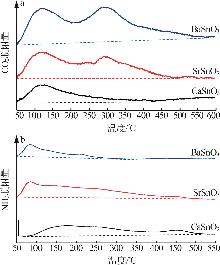
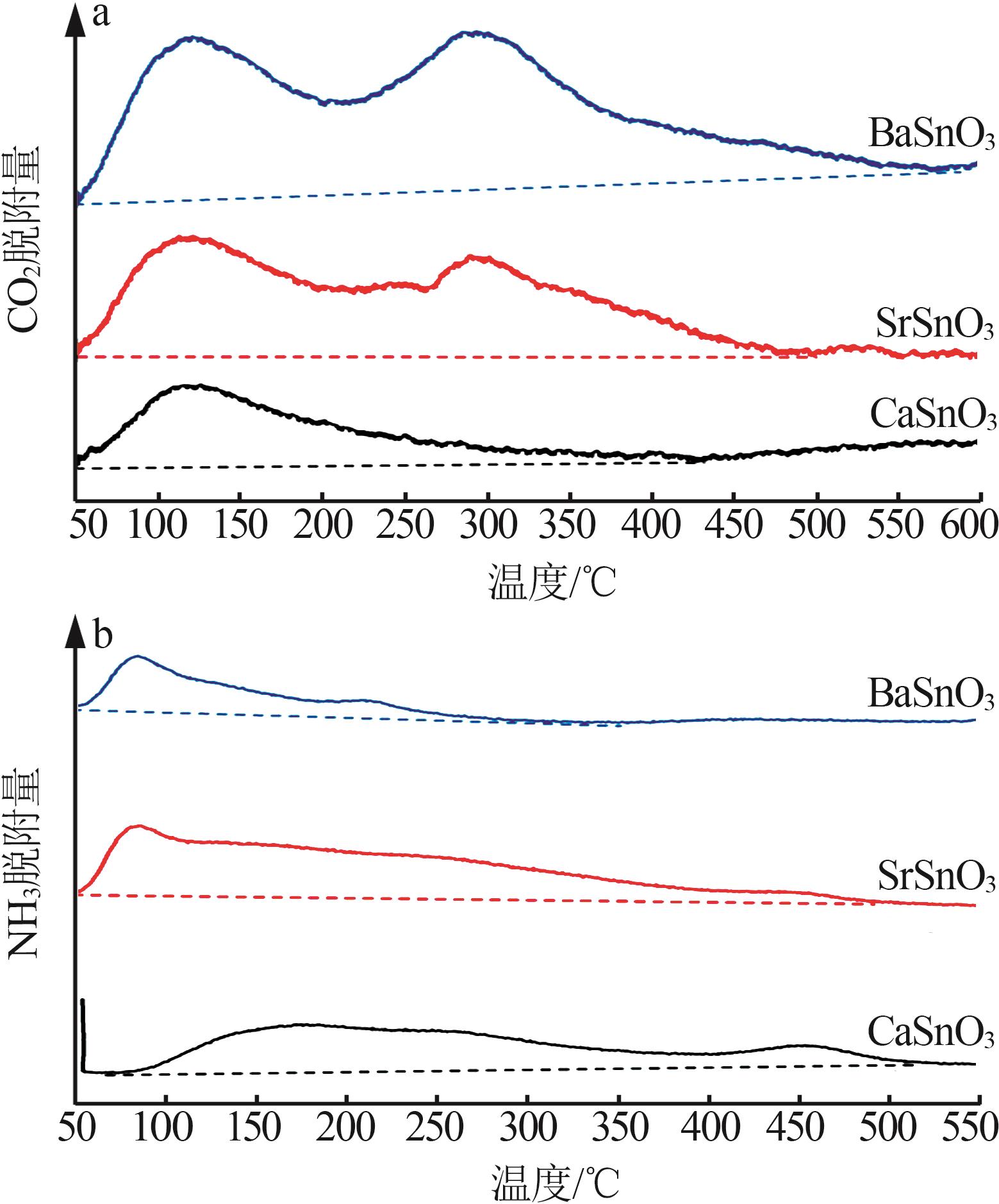
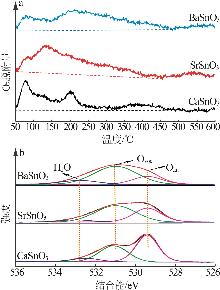
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (4): 37-44.doi: 10.19964/j.issn.1006-4990.2024-0199
• Research & Development • Previous Articles Next Articles
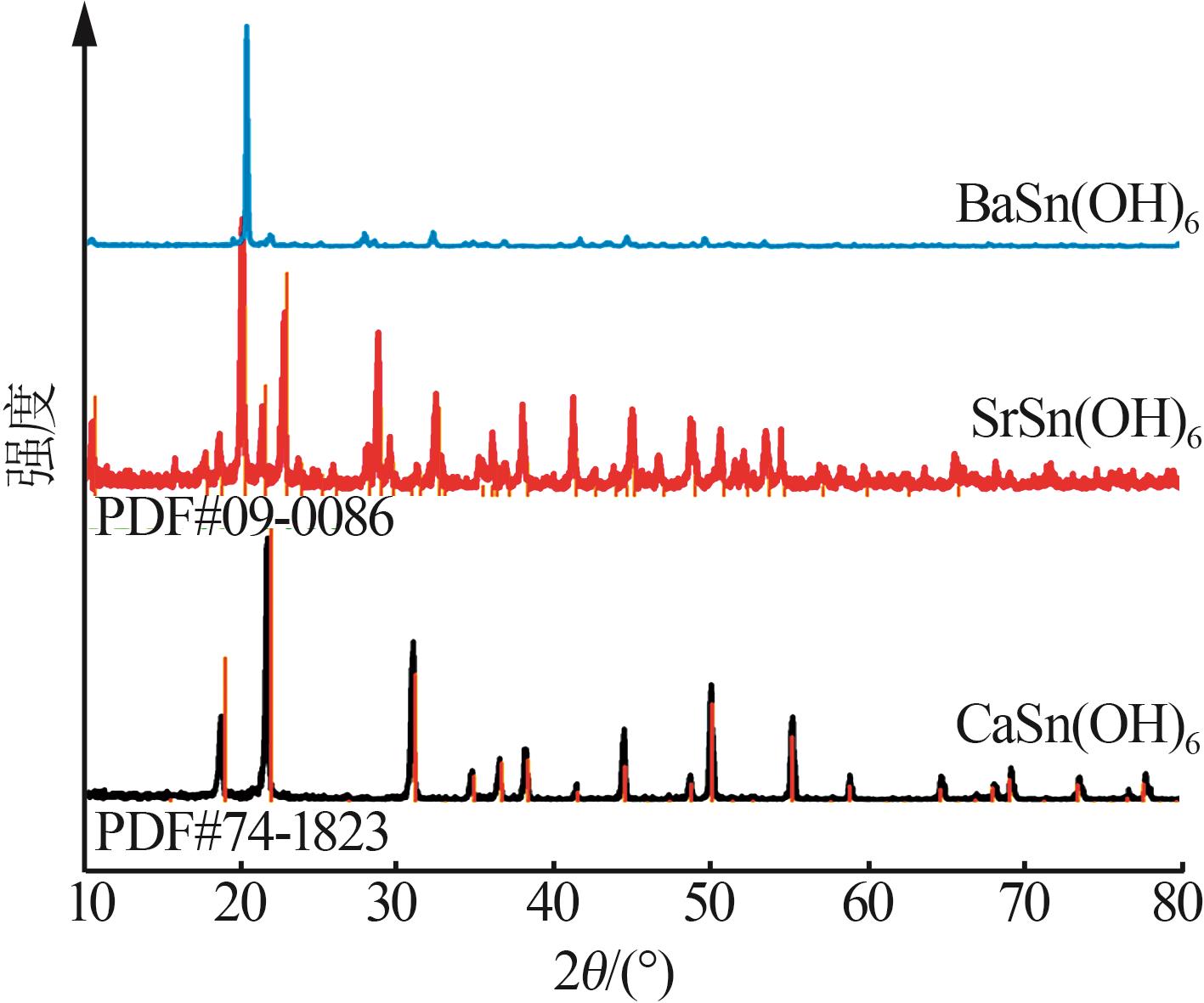
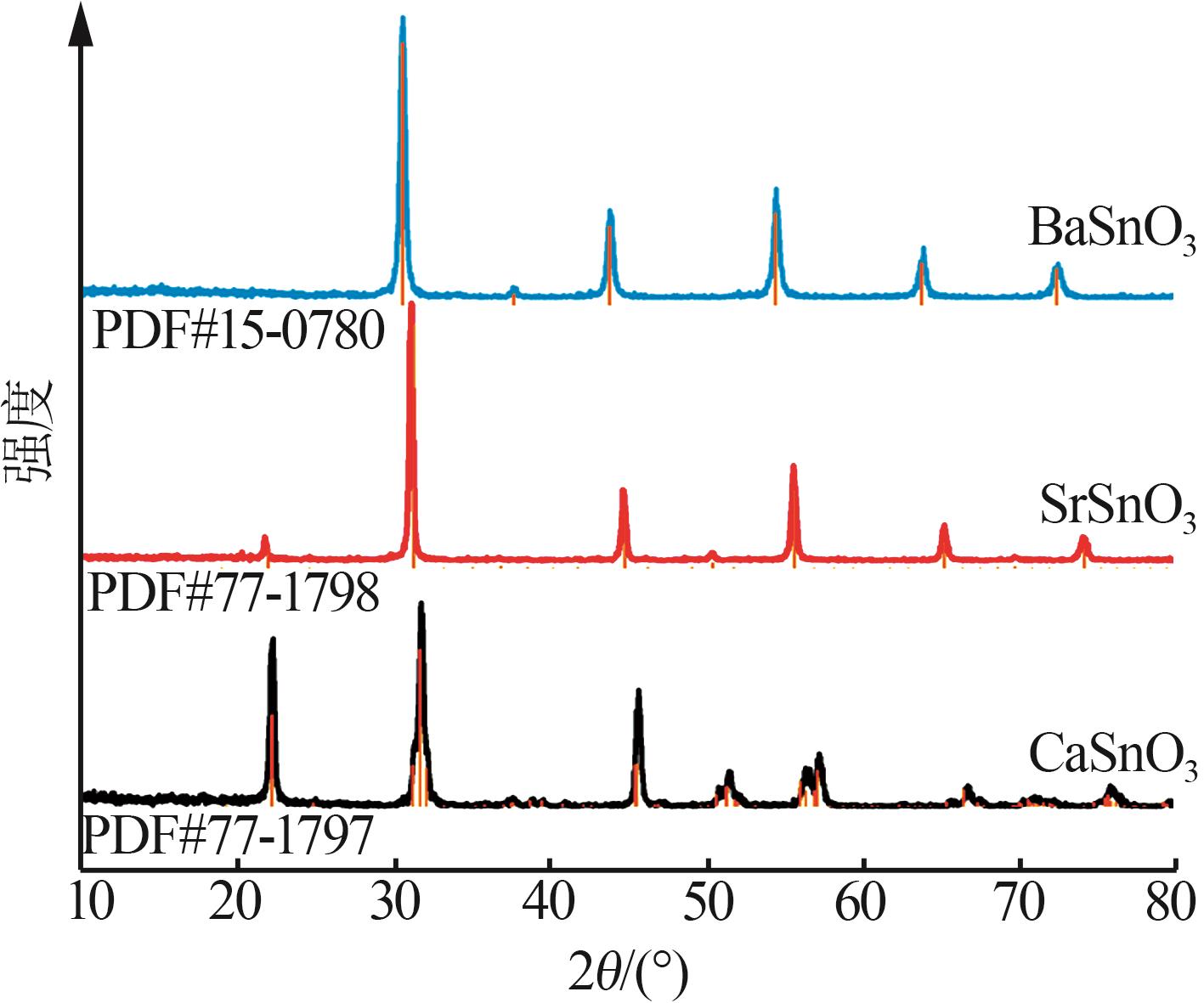
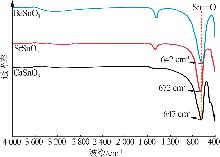
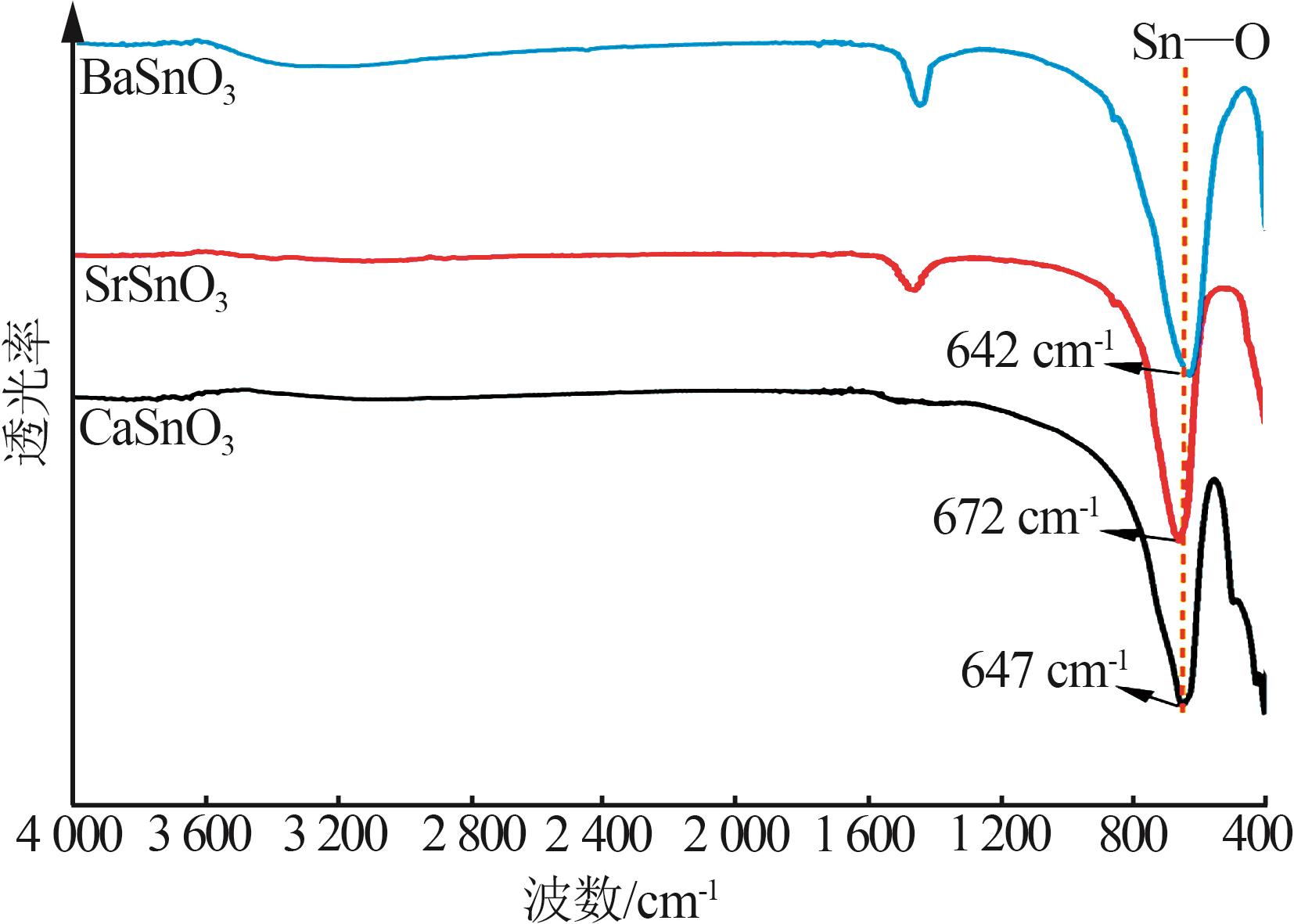
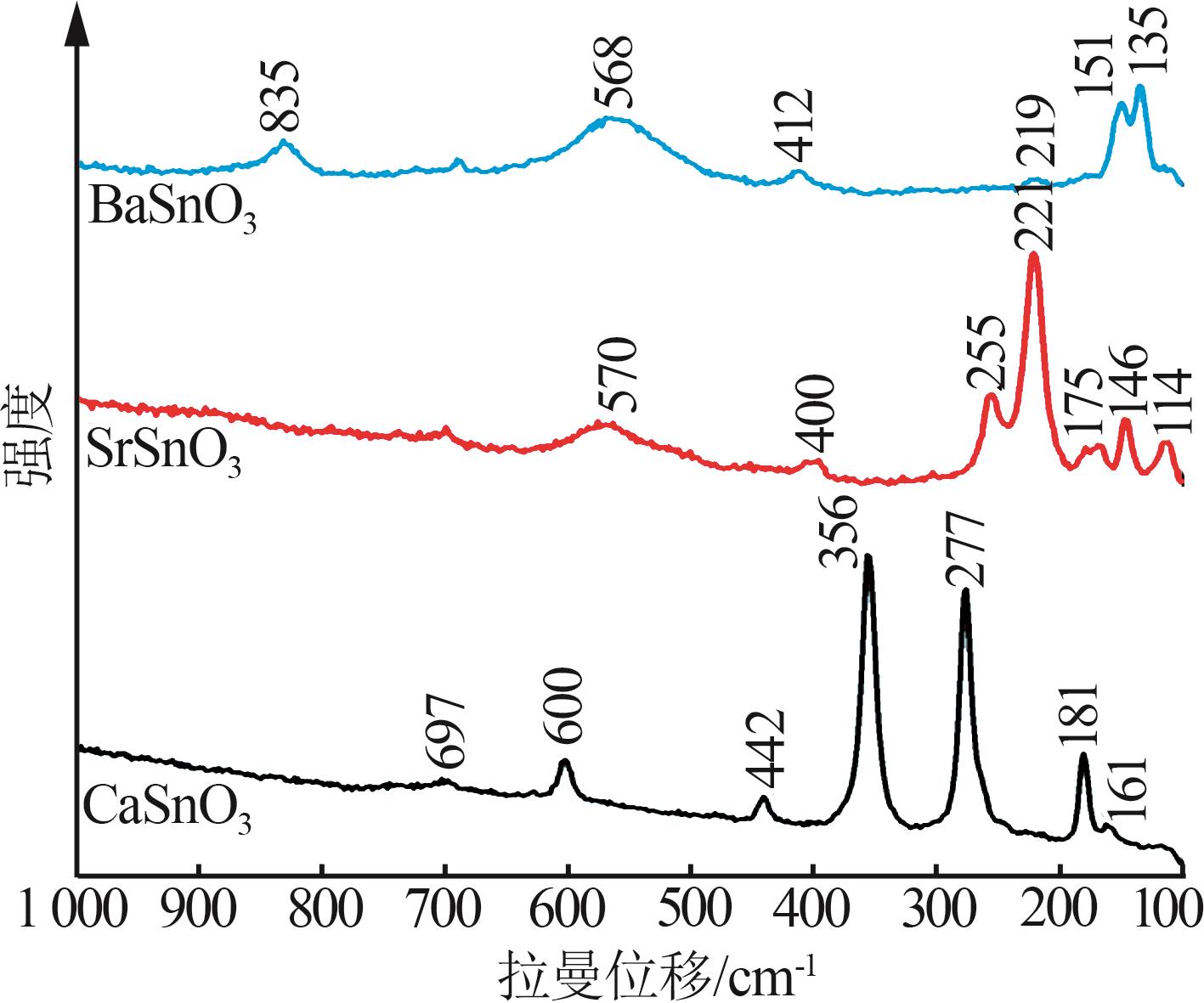
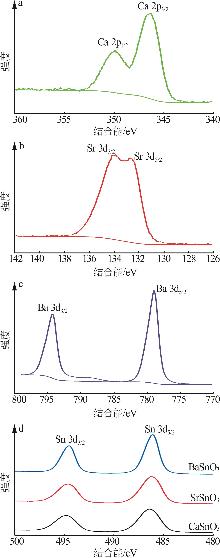
Hydrothermal synthesis of tin-based perovskite and study on high-temperature crystalline phase transition and surface active sites
- 1. Nanchang Medical College,Nanchang 330004,China
2. Jiangxi Provincial Academy of Sciences,Institute of Applied Chemistry,Nanchang 330096,China
-
Received:2024-04-09Online:2025-04-10Published:2025-04-21 -
Contact:XU Junwei E-mail:602023040002@smail.nju.edu.cn
CLC Number:
Cite this article
CHEN Guanyi, XU Junwei. Hydrothermal synthesis of tin-based perovskite and study on high-temperature crystalline phase transition and surface active sites[J]. Inorganic Chemicals Industry, 2025, 57(4): 37-44.
share this article
| [1] | ARTINI C.Crystal chemistry,stability and properties of interlanthanide perovskites:A review[J].Journal of the European Ceramic Society,2017,37(2):427-440. |
| [2] | LI Hanxi, LAI Cui, WEI Zhen,et al.Strategies for improving the stability of perovskite for photocatalysis:A review of recent progress[J].Chemosphere,2023,344:140395. |
| [3] | JIANG Mingquan, LU Junhan, CHEN Yi,et al.Non-hydrolytic sol-gel synthesis of BaTiO3 nano-powder:Effect of calcination temperature and modified titanium source on particles[J].Materials Today Communications,2024,38:108494. |
| [4] | MISHRA G, MINOR C, TIWARI A.High throughput synthesis of BaSnO3 microcrystals by molten salt technique[J].Materials Chemistry and Physics,2023,295:127042. |
| [5] | BIKYASHEV E A, KUBRIN S P, POPOV A V,et al.Evolution of the structure of MSnO3(M=Ba,Sr) perovskites during hydrothermal synthesis and their photocatalytic activity[J].Dalton Transactions,2023,52(47):17881-17893. |
| [6] | Masoumeh H, Mojgan G, Elmuez A D,et al.CaSnO3/g-C3N4 S-scheme heterojunction photocatalyst for the elimination of erythrosine and eriochrome black T from water under visible light[J].Results in Engineering,2024,21:101903. |
| [7] | MURALIDHARAN M, SELVAKUMAR S, SIVAKUMAR K,et al.Effect of Yb doping on structural,optical and induced ferromagnetism in SrSnO3 perovskite nanostructures[J].Physica B:Condensed Matter,2021,615:413039. |
| [8] | ASISI JANIFER M, ANAND S, JOSEPH PRABAGAR C,et al.Structural and optical properties of BaSnO3 ceramics by solid state reaction method[J].Materials Today:Proceedings,2021,47:2067-2070. |
| [9] | MANOHARAN A, SURESH B, AJAY K,et al.Mixed ferro to diamagnetic transition in Hf doped CaSnO3 perovskite system[J].Solid State Communications,2023,360:115033. |
| [10] | MURALIDHARAN M, AJAYKUMARI P, AVINASH M,et al.Optical,magnetic and defect studies of Ni2+ doped SrSnO3 nanostructures[J].Ceramics International,2024,50(8):12840-12851. |
| [11] | ZHANG Weifeng, TANG Junwang, YE Jinhua.Structural,photocatalytic,and photophysical properties of perovskite MSnO3(M=Ca,Sr,and Ba) photocatalysts[J].Journal of Materials Research,2007,22(7):1859-1871. |
| [12] | SHRIVASTAVA S, NEHRA S, KUMAR S,et al.Temperature-dependent dielectric measurements and structural properties of barium stannate(BaSnO3)[J].Journal of Alloys and Compounds,2023,957:170458. |
| [13] | MURALEEDHARAN S, ASHOK A M.Nitrogen doping for effective enhancement of optoelectronic performance of ASnO3(A-Ca,Sr,Ba) transparent conducting oxides deposited by doctor blade method[J].Optik,2023,287:171096. |
| [14] | 李小强,陈欣,李洪波,等.碳包覆CaSnO3纳米纤维作为高性能锂离子电池负极材料[J].无机化学学报,2021,37(4):700-708. |
| LI Xiaoqiang, CHEN Xin, LI Hongbo,et al.Carbon-coated CaSnO3 nanofibers as high performance anode materials for lithium ion batteries[J].Chinese Journal of Inorganic Chemistry,2021,37(4):700-708. | |
| [15] | CHANTELLE L, KENNEDY B J, DE OLIVEIRA C P,et al.Europium induced point defects in SrSnO3-based perovskites employed as antibacterial agents[J].Journal of Alloys and Compounds,2023,956:170353. |
| [16] | CHU Xiangfeng, GAN Zhengqiang, BAI Linshan,et al.The acetic acid vapor sensing properties of BaSnO3 microtubes prepared by electrospinning method[J].Materials Science and Engineering:B,2020,259:114606. |
| [17] | GLERUP M, KNIGHT K S, POULSEN F W.High temperature structural phase transitions in SrSnO3 perovskite[J].Materials Research Bulletin,2005,40(3):507-520. |
| [18] | SINGH PAL R, RANA S, KUMAR SHARMA S,et al.Enhancement of oxygen vacancy sites of La2- x M x Ce2O7- δ (M=Ca,Ba,Sr) catalyst for the low temperature oxidative coupling of methane:A combined DFT and experimental study[J].Chemical Engineering Journal,2023,458:141379. |
| [19] | CHOUDHARY V R, RANE V H.Acidity/basicity of rare-earth oxides and their catalytic activity in oxidative coupling of methane to C2-hydrocarbons[J].Journal of Catalysis,1991,130(2):411-422. |
| [20] | 黄学辉,夏莹,张丽丽,等.纳米类钙钛矿化合物La4BaCu5- x- y Fe x Co y O13+ δ 的制备及表征[J].陶瓷学报,2010,31(4):561-564. |
| HUANG Xuehui, XIA Ying, ZHANG Lili,et al.Prepar-ation and characterization of perovskite-like compounds La 4 BaCu5- x- y Fe x Co y O13+ δ with doping in the "B" position[J].Journal of Ceramics,2010,31(4):561-564. | |
| [21] | FAISAL M, HARRAZ F A, ISMAIL A A,et al.Novel synthesis of polyaniline/SrSnO3 nanocomposites with enhanced photocatalytic activity[J].Ceramics International,2019,45(16):20484-20492. |
| [22] | WANG Menghui, ZHU Changzu, HU Huashuai,et al.Correlation between radiation resistance and structural factors of ABO3-type perovskites[J].Nuclear Instruments and Methods in Physics Research Section B:Beam Interactions with Materials and Atoms,2023,536:88-96. |
| [23] | GAO Wanlin, ZHOU Tuantuan, WANG Qiang.Controlled synthesis of MgO with diverse basic sites and its CO2 capture mechanism under different adsorption conditions[J].Chemical Engineering Journal,2018,336:710-720. |
| [24] | FRAILE J M, GARCÍA N, MAYORAL J A,et al.The influence of alkaline metals on the strong basicity of Mg-Al mixed oxides:The case of transesterification reactions[J].Applied Catalysis A:General,2009,364(1/2):87-94. |
| [25] | KUŚ S, OTREMBA M, TÓRZ A,et al.Further evidence of responsibility of impurities in MgO for variability in its basicity and catalytic performance in oxidative coupling of methane[J].Fuel,2002,81(13):1755-1760. |
| [26] | COOK R L, SAMMELLS A F.On the systematic selection of perovskite solid electrolytes for intermediate temperature fuel cells[J].Solid State Ionics,1991,45(3/4):311-321. |
| [1] | LI Zihan, ZHANG Jiaqi, LI Shizhuo, LI Xinyu, LIU Shaozhuo, WANG Yihao, HAO Yucui, LIU Jian, LI Yanhua. Study on synthesis and catalytic mechanism of CdS/g-C3N4 composite photocatalyst [J]. Inorganic Chemicals Industry, 2025, 57(3): 124-132. |
| [2] | WANG Yawen, WANG Fangfang, GENG Siyu, JU Jia, CHEN Lei, CHEN Changdong. Study on preparation and photocatalytic performance of SrTiO3-SrWO4 [J]. Inorganic Chemicals Industry, 2024, 56(7): 143-149. |
| [3] | DI Lu, WANG Weiguo, CHEN Juexian, WU Chuanshu. Study on preparation of transition metal-supported Silicalite-1 zeolite catalyst and its catalytic performance for furfural hydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(4): 125-132. |
| [4] | ZHAN Sijin, LIU Shike, LIU Fei, YAO Mengqin, CAO Jianxin. Study on preparation and catalytic performance of ZnO-CeO2 [J]. Inorganic Chemicals Industry, 2024, 56(3): 137-143. |
| [5] | REN Qixia, YANG Kun, LIU Fei, YAO Mengqin, CAO Jianxin. Effect of promoter on physicochemical properties and catalytic performance of ZnO/ZrO2 [J]. Inorganic Chemicals Industry, 2024, 56(3): 144-154. |
| [6] | YANG Kun, REN Qixia, DONG Yonggang, LIU Fei, YAO Mengqin, CAO Jianxin. Effect of calcination temperature on catalytic performance of ZnGaZrO x /SAPO-34 [J]. Inorganic Chemicals Industry, 2024, 56(2): 136-145. |
| [7] | WANG Ruirui, ZHU Chaoliang, MU Bing, MA Wanxia, FAN Jie, XU Guowang, SHI Yifei, DENG Xiaochuan, QING Binju. Preparation of cubic manganese carbonate by hydrothermal method and its application in extraction of lithium [J]. Inorganic Chemicals Industry, 2024, 56(12): 94-103. |
| [8] | CHEN Zhangxu, FU Minglian, ZHU Danchen, ZHENG Bingyun. Preparation of carbon/graphite carbon nitride composites and their methylene blue removal performance [J]. Inorganic Chemicals Industry, 2023, 55(3): 134-139. |
| [9] | JIANG Demin, LI Shunmei, Li Qingqing, Chen Yuxin, LIU Daijun. Hydrothermal synthesis of basic magnesium sulfate whiskers from magnesium hydroxide and magnesium sulfate heptahydrate [J]. Inorganic Chemicals Industry, 2023, 55(12): 74-81. |
| [10] | YANG Tinglong,WANG Fuzhong,LIU Fei. Study on sulfur poisoning of zirconium-based bimetallic oxides catalyst [J]. Inorganic Chemicals Industry, 2023, 55(1): 151-158. |
| [11] | LU Yang,WANG Jing,HE Lijie,WANG Fudong,ZHANG Jiaming. Research progress on preparation of magnesium aluminate spinel powders and it in field of luminescent material [J]. Inorganic Chemicals Industry, 2022, 54(9): 39-46. |
| [12] | LI Xiao,ZHAO Yuxiang,LI Bo,ZOU Xingwu,WANG Shuxuan. Study on controllable synthesis of spherical strontium fluoride [J]. Inorganic Chemicals Industry, 2022, 54(5): 67-71. |
| [13] | LU Zheng,CHEN Kunfeng,XUE Dongfeng. Study on large-scale preparation and electrochemical properties of high thermal stabilized α-Fe2O3 [J]. Inorganic Chemicals Industry, 2022, 54(3): 45-50. |
| [14] | PEI Yinchang,MO Shengpeng,XIE Qinglin,CHEN Nanchun. Study on mechanism of zeolite A synthesized from stellerite zeolite by two-step hydrothermal method [J]. Inorganic Chemicals Industry, 2022, 54(11): 59-64. |
| [15] | QI Yuanhao,WU Jinxiu,LIU Zhaogang,HU Yanhong,FENG Fushan,LI Jianfei,WANG Xin,LIU Conglin. Preparation and characterization of anhydrous calcium sulfate whisker [J]. Inorganic Chemicals Industry, 2022, 54(10): 109-115. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||