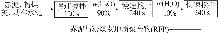Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (11): 83-90.doi: 10.19964/j.issn.1006-4990.2024-0521
• Environment·Health·Safety • Previous Articles Next Articles
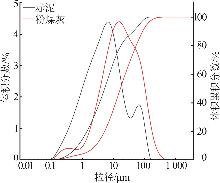
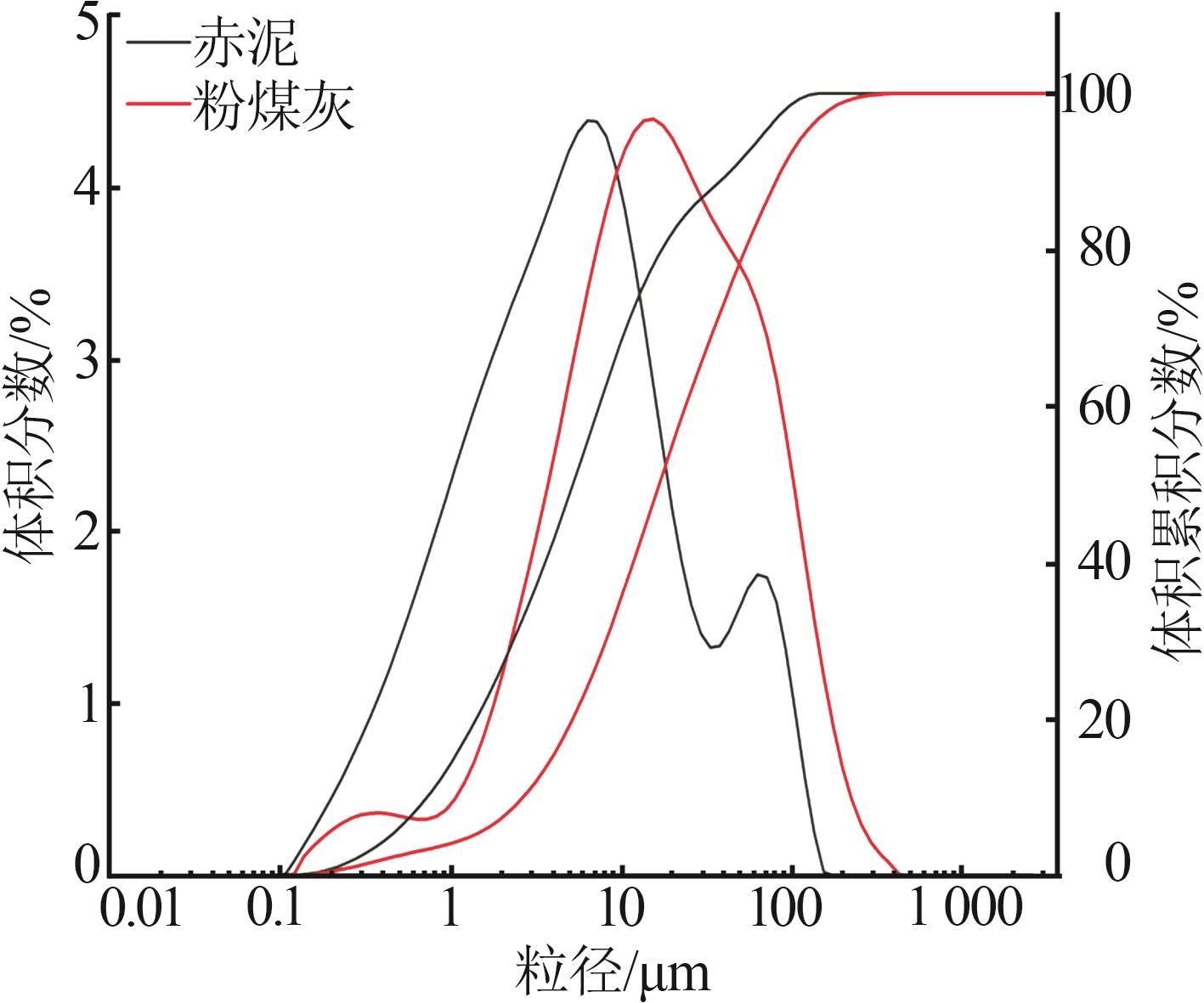
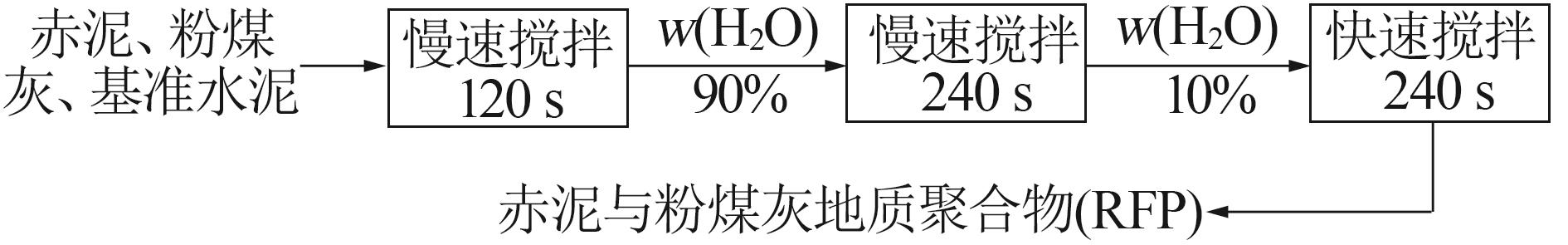
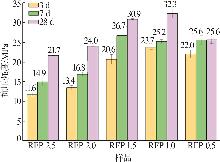
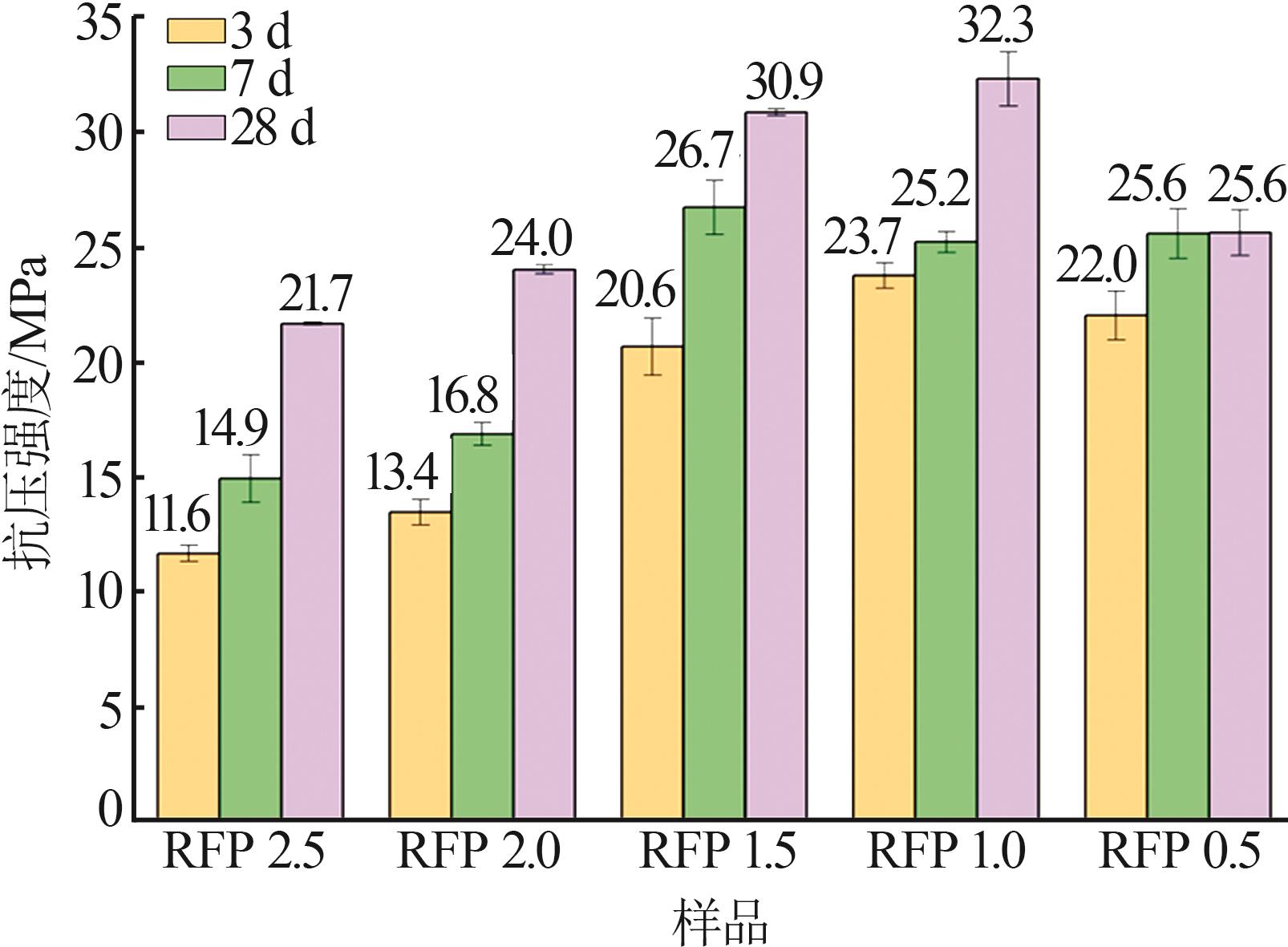
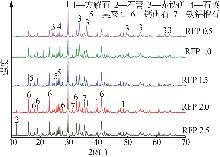
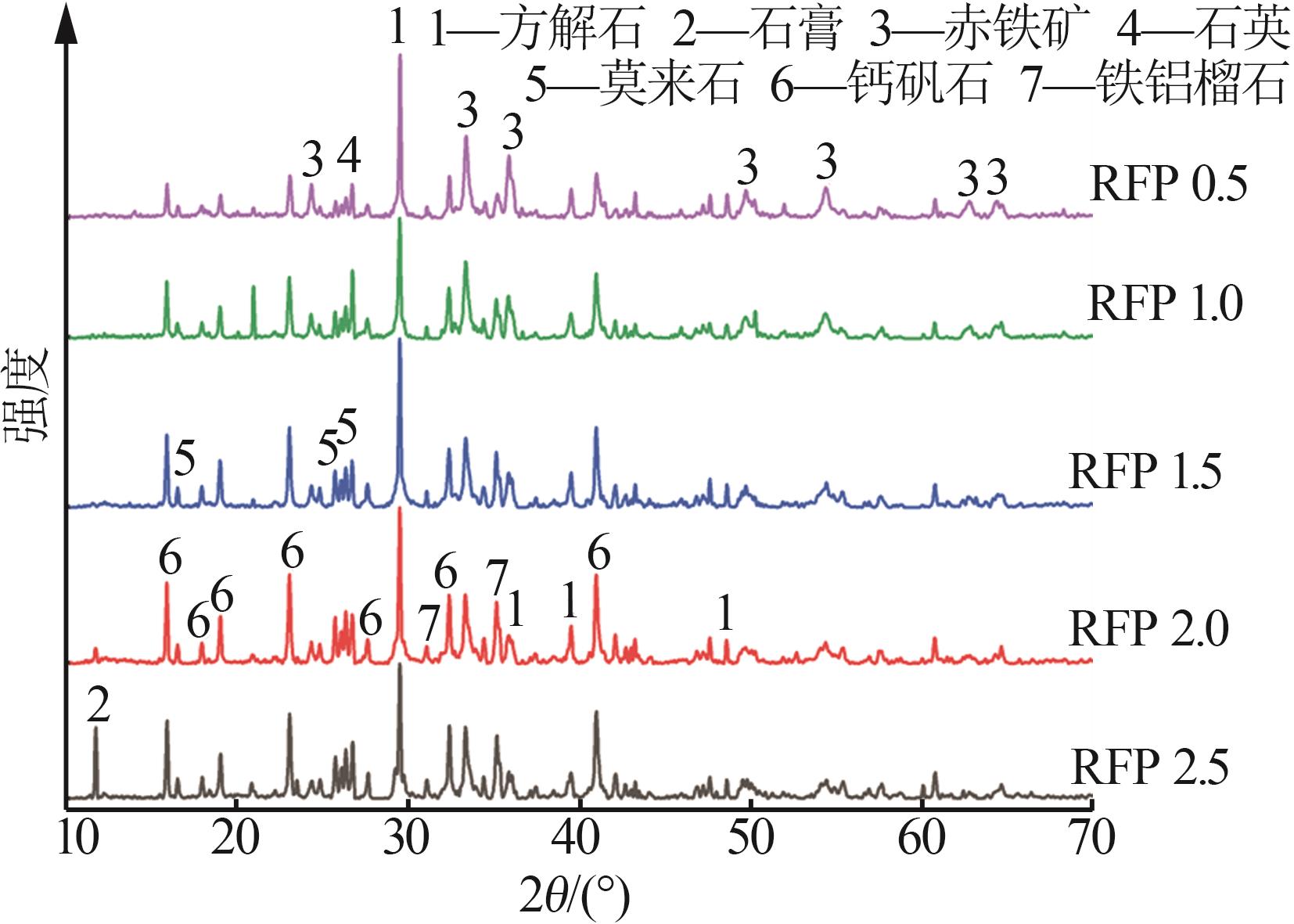
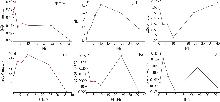
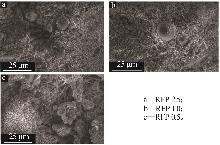
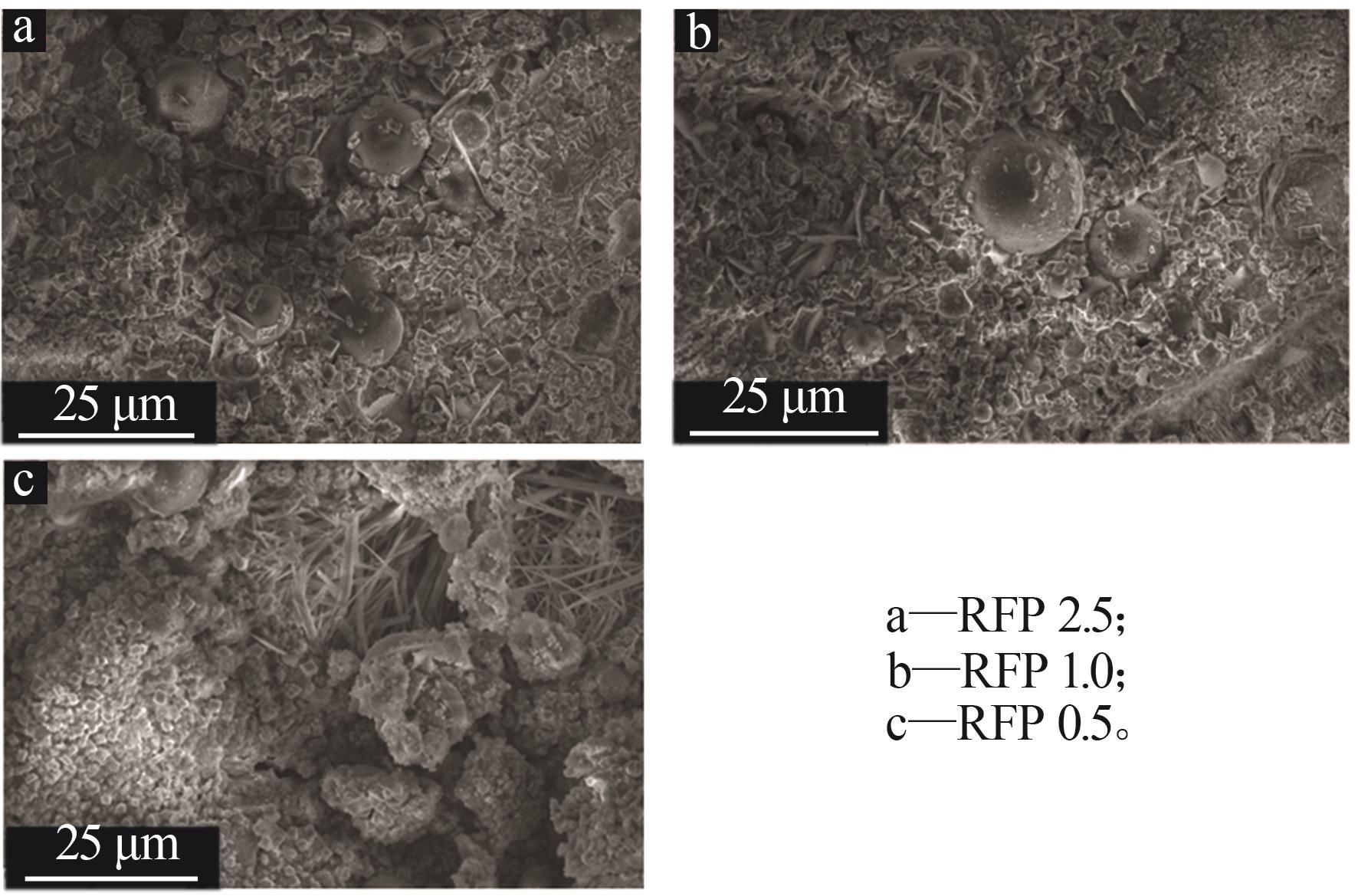
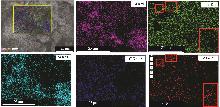
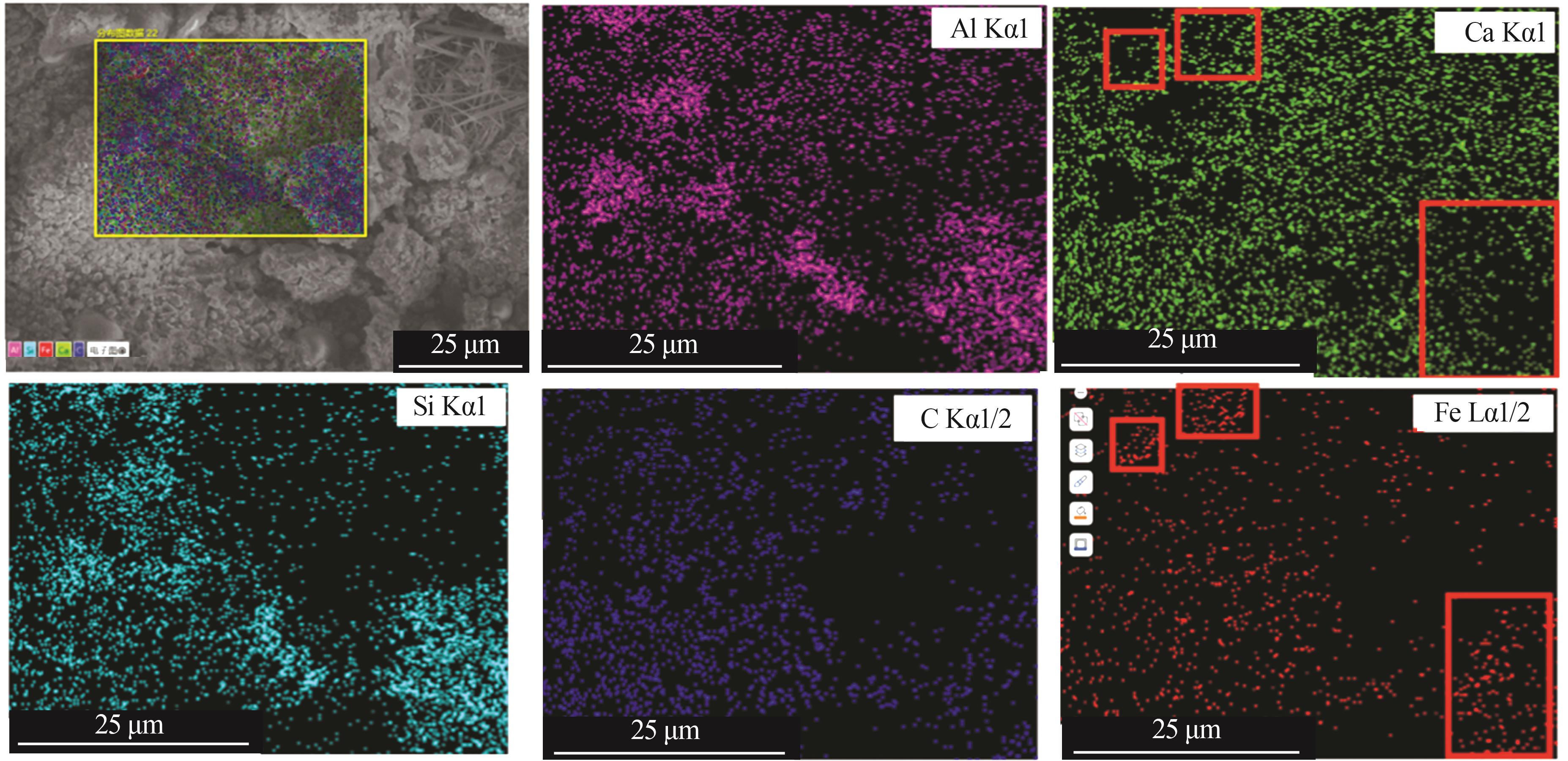
Study on early hydration and mechanism of Bayer red mud and fly ash geopolymer
LI Changjiang( ), GUAN Xuemao(
), GUAN Xuemao( ), LIU Xiaoxing, ZHANG Neng, LUO Jintao
), LIU Xiaoxing, ZHANG Neng, LUO Jintao
- School of Materials Science and Engineering,Henan Polytechnic University,Jiaozuo 454003,China
-
Received:2024-09-30Online:2025-11-10Published:2025-01-06 -
Contact:GUAN Xuemao E-mail:360041086@qq.com;guanxuemao@hpu.edu.cn
CLC Number:
Cite this article
LI Changjiang, GUAN Xuemao, LIU Xiaoxing, ZHANG Neng, LUO Jintao. Study on early hydration and mechanism of Bayer red mud and fly ash geopolymer[J]. Inorganic Chemicals Industry, 2025, 57(11): 83-90.
share this article
| [1] | LIU Qing, CUI Mingyao, LI Xiaochang,et al.Alkali-hydrothermal activation of mine tailings to prepare one-part geopolymer:Activation mechanism,workability,strength,and hydration reaction[J]. Ceramics International,2022,48(20):30407-30417. |
| [2] | LI Zhaofeng, YOU Hao, GAO Yifan,et al.Effect of ultrafine red mud on the workability and microstructure of blast furnace slag-red mud based geopolymeric grouts[J].Powder Technology,2021, 392:610-618. |
| [3] | MURALEEDHARAN M, NADIR Y.Factors affecting the mechanical properties and microstructure of geopolymers from red mud and granite waste powder:A review[J].Ceramics International,2021,47(10):13257-13279. |
| [4] | WANG Qingwei, HAN Song, YANG Junhao,et al.Preparation of geopolymer concrete with Bayer red mud and its reaction mechanism[J].Construction and Building Materials,2023,409:133730. |
| [5] | NORDHEIM E, BARRASSO G.Sustainable development indicators of the European aluminium industry[J].Journal of Cleaner Production,2007,15(3):275-279. |
| [6] | 胡明慧,郄志鹏,张新翰,等.碳中和背景下的赤泥综合利用现状[J].有色金属(冶炼部分),2024(3):69-75. |
| HU Minghui, Zhipeng QIE, ZHANG Xinhan,et al.Status of comprehensive utilization of red mud in the context of carbon neutrality[J].Nonferrous Metals(Extractive Metallurgy),2024(3):69-75. | |
| [7] | BAI Bing, BAI Fan, NIE Qingke,et al.A high-strength red mud-fly ash geopolymer and the implications of curing temperature[J].Powder Technology,2023,416:118242. |
| [8] | SINGH V, BANO S, CHAUHAN V B,et al.Red mud as a sustainable road construction material:An experimental investigation[J].Construction and Building Materials,2024,411:134549. |
| [9] | PATANGIA J, SARAVANAN T J, KABEER K I S A,et al.Study on the utilization of red mud(bauxite waste) as a supplementary cementitious material:Pathway to attaining sustainable development goals[J].Construction and Building Materials,2023,375:131005. |
| [10] | PANGDAENG S, PHOO-NGERNKHAM T, SATA V,et al.Influence of curing conditions on properties of high calcium fly ash geopolymer containing Portland cement as additive[J].Materials & Design,2014,53:269-274. |
| [11] | 王茹霆,赵小蓉,黄绪泉,等.混合相磷石膏基胶结材制备与早期性能研究[J].无机盐工业,2024,56(3):98-104,154. |
| WANG Ruting, ZHAO Xiaorong, HUANG Xuquan,et al.Research on preparation and early performance of mixed phase phosphogypsum-based cementing materials[J].Inorganic Chemicals Industry,2024,56(3):98-104,154. | |
| [12] | GONÇALVES N P F, OLHERO S M, LABRINCHA J A,et al.3D-printed red mud/metakaolin-based geopolymers as water pollutant sorbents of methylene blue[J].Journal of Cleaner Production,2023,383:135315. |
| [13] | ZHENG Xianrong, TIAN Guangyuan, LI Yongjia.Study on desulfurization performance of Cu modified red mud-fly ash sorbent[J].Journal of Environmental Chemical Engineering,2023,11(5):110892. |
| [14] | XIE Jun, HE Bin, CHEN Tao,et al.Characterization of red mud and fly ash based for co-curing of Cu2+-contaminated soil[J].Case Studies in Construction Materials,2024,20:e02769. |
| [15] | CHEN Tao, WANG Linhao, HE Bin,et al.Study on the solidification/stabilization of cadmium-contaminated soil by red mud-assisted blast furnace slag under excitation conditions[J].Journal of Cleaner Production,2024,435:140505. |
| [16] | ÖZCAN M, BIROL B, KAYA F.Investigation of photocatalytic properties of TiO2 nanoparticle coating on fly ash and red mud based porous ceramic substrate[J].Ceramics International,2021,47(17):24270-24280. |
| [17] | UYSAL M, AYGÖRMEZ Y, CANPOLAT O,et al.Investigation of using waste marble powder,brick powder,ceramic powder,glass powder,and rice husk ash as eco-friendly aggregate in sustainable red mud-metakaolin based geopolymer composites[J].Construction and Building Materials,2022,361:129718. |
| [18] | PAN Xiaolin, WU Hongfei, LV Zhongyang,et al.Recovery of valuable metals from red mud:A comprehensive review[J].Science of the Total Environment,2023,904:166686. |
| [19] | 李扬,徐博,杨赫,等.Cu/Ce负载对赤泥脱除中低温烟气中NO的促进作用[J].燃料化学学报(中英文),2024,52(3):362-372. |
| LI Yang, XU Bo, YANG He,et al.Promotion of Cu/Ce supported red mud for NO removal from low and medium temperature flue gas[J].Journal of Fuel Chemistry and Technology,2024,52(3):362-372. | |
| [20] | 赵恒,李望,牛泽鹏,等.赤泥制备聚合硫酸铝铁及其处理废水的研究[J].材料导报,2020,34(21):21038-21044. |
| ZHAO Heng, LI Wang, NIU Zepeng,et al.Study on synthesis of PAFS based on red mud and its application in wastewater treatment[J].Materials Reports,2020,34(21):21038-21044. | |
| [21] | 鲁镜镜,谢燕,李宸,等.载镧磁化赤泥处理含磷废水的研究[J].无机盐工业,2023,55(2):99-105. |
| LU Jingjing, XIE Yan, LI Chen,et al.Study on treatment of phosphorus?containing wastewater by lanthanum?loaded magnetized red mud[J].Inorganic Chemicals Industry,2023,55(2):99-105. | |
| [22] | 陶敏龙,张召述,卓瑞锋.利用赤泥制备CBC复合材料的研究[J].有色金属(冶炼部分),2009(4):45-48. |
| TAO Minlong, ZHANG Zhaoshu, ZHUO Ruifeng.Study on the CBC compoisite material made from red mud[J].Nonferrous Metals (Extractive Metallurgy),2009(4):45-48. | |
| [23] | YAO Yuan, LI Yu, LIU Xiaoming,et al.Characterization on a cementitious material composed of red mud and coal industry byproducts[J].Construction and Building Materials,2013,47:496-501. |
| [24] | 侯双明,高嵩,张蕾,等.热活化和机械活化对拜耳法赤泥性能影响[J].硅酸盐通报,2020,39(5):1573-1577. |
| HOU Shuangming, GAO Song, ZHANG Lei,et al.Effects of thermal and mechanical activation on properties of bayer red mud[J].Bulletin of the Chinese Ceramic Society,2020,39(5):1573-1577. | |
| [25] | YE Nan, YANG Jiakuan, LIANG Sha,et al.Synthesis and strength optimization of one-part geopolymer based on red mud[J].Construction and Building Materials,2016,111:317-325. |
| [26] | 王晶.赤泥基胶凝材料的制备及性能研究[D].西安:西安建筑科技大学,2016. |
| WANG Jing.Study on preparation and performance of red mud based cementitious material[D].Xi′an :Xi′an University of Architecture and Technology,2016. | |
| [27] | 刘娟红,周在波,吴爱祥,等.低浓度拜耳赤泥充填材料制备及水化机理[J].工程科学学报,2020,42(11):1457-1464. |
| LIU Juanhong, ZHOU Zaibo, WU Aixiang,et al.Preparation and hydration mechanism of low concentration Bayer red mud filling materials[J].Chinese Journal of Engineering,2020,42(11):1457-1464. | |
| [28] | HU Wei, NIE Qingke, HUANG Baoshan,et al.Mechanical and microstructural characterization of geopolymers derived from red mud and fly ashes[J].Journal of Cleaner Production,2018,186:799-806. |
| [29] | 刘晓明,张增起,李宇,等.赤泥在建筑材料和复合高分子材料中的利用研究进展[J].材料导报,2023,37(10):15-28. |
| LIU Xiaoming, ZHANG Zengqi, LI Yu,et al.Research progress of utilization of red mud in building materials and geopolymer composites[J].Materials Reports,2023,37(10):15-28. | |
| [30] | CHOO H,LIM S, LEE W,et al.Compressive strength of one-part alkali activated fly ash using red mud as alkali supplier[J].Construction and Building Materials,2016,125:21-28. |
| [31] | MUDGAL M, SINGH A, CHOUHAN R K,et al.Fly ash red mud geopolymer with improved mechanical strength[J].Cleaner Engineering and Technology,2021,4:100215. |
| [32] | HE Jingyuan, GUO Tao, LI Zhaofeng,et al.Hydration mechanism of red mud-fly ash based geopolymer[J].Materials Chemistry and Physics,2024,314:128807. |
| [33] | LI Jiangchuan, CHANG Jun, WANG Tong,et al.Effects of phosphogypsum on hydration properties and strength of calcium aluminate cement[J].Construction and Building Materials,2022,347:128398. |
| [34] | BAI Ru, ZHANG Ju, YAN Changwang,et al.Calcium hydroxide content and hydration degree of cement in cementitious composites containing calcium silicate slag[J].Chemosphere,2021,280:130918. |
| [35] | TEUNE I E, SCHOLLBACH K, FLOREA M V A,et al.Carbonation of hydrated cement:The impact of carbonation conditions on CO2 sequestration,phase formation,and reactivity[J].Journal of Building Engineering,2023,79:107785. |
| [36] | LABHASETWAR N K, SHRIVASTAVA O P, MEDIKOV Y Y.Mo¨ssbauer study on iron-exchanged calcium silicate hydrate:Ca5- x Fe x Si6O18H2·nH2O[J].Journal of Solid State Chemistry,1991,93(1):82-87. |
| [37] | LIU Xin, FENG Pan, CHEN Jin,et al.A critical review on the interaction between calcium silicate hydrate(C-S-H) and different ions[J].Construction and Building Materials,2024,413:134931. |
| [38] | DU Zhaowen, SHENG Shouqian, GUO Jiaxing.Effect of composite activators on mechanical properties,hydration activity and microstructure of red mud-based geopolymer[J].Journal of Materials Research and Technology,2023,24:8077-8085. |
| [39] | LIU Xiao, ZHOU Wentao, GUAN Xiangai.Thermal analysis and isothermal reduction kinetics of alkaline hematite in an advanced suspension roaster[J].Separation and Purification Technology,2023,323:124493. |
| [40] | FAUCON P, LE BESCOP P, ADENOT F,et al.Leaching of cement:Study of the surface layer[J].Cement and Concrete Research,1996,26(11):1707-1715. |
| [41] | FANG Yuan, ZHUANG Kunde, CUI Hongzhi,et al.The state of Fe3+ in the C-F-A-S-H system with varying Fe/Si and Ca/Si ratios[J].Journal of Materials Chemistry A,2023,11(47):26193-26211. |
| [1] | WANG Feng, SONG Yu, HE Zhaoyi, XIA Yuhua, BI Yanli, FENG Hao, CAO Dongwei. Study on mechanical properties and microscopic characterization of phosphogypsum composite cementitious materials [J]. Inorganic Chemicals Industry, 2025, 57(5): 93-99. |
| [2] | LIU Xinlong, YANG Zhenyu, HAO He, LIU Shuxin, WU Chenyang, WANG Xingli, MA Qingqing. Study on shaped 4A zeolite synthesized with aluminum extraction residue by fly ash [J]. Inorganic Chemicals Industry, 2025, 57(3): 78-85. |
| [3] | LI Chao, WANG Liping, GAO Guimei, ZHANG Yunfeng, HONG Yu, LIU Darui, XU Lijun, CUI Yongjie. Study on reaction mechanism of acid leaching lithium from circulating fluidized bed fly ash [J]. Inorganic Chemicals Industry, 2025, 57(3): 101-107. |
| [4] | FENG Hao, QIN Meng, HE Zhaoyi, BI Yanli, SONG Yu, DU Youcheng. Effect of silica fume and fly ash on properties of slag-based geopolymer [J]. Inorganic Chemicals Industry, 2025, 57(11): 91-99. |
| [5] | ZHAO Feiyan, ZHANG Xiaodong, DU Yanxia, WANG Qiang, LI Xiaoyan. Preparation technology and research progress of fly ash ceramsite [J]. Inorganic Chemicals Industry, 2024, 56(4): 16-23. |
| [6] | LI Kuai, LI Zhaoshuai, DONG Tingxuan, LI Dan, GUO Shengwei, HAN Fenglan. Study on effect of wet magnetic separation on distribution of Fe and heavy metal in fly ash [J]. Inorganic Chemicals Industry, 2024, 56(4): 98-104. |
| [7] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [8] | NI Dong, TANG Liang, HE Zhaoyi, WANG Jian, PEI Shanshan, XIA Lei. Study on sulfate activation performance of electrolytic manganese residue in hydrated lime-slag system [J]. Inorganic Chemicals Industry, 2024, 56(11): 151-157. |
| [9] | ZHAO Shiyong, XIAO Yuchen, MA Qingqing, YANG Zhenni, WANG Jizhen, FAN Xiaoping. Study on adsorption of Cu(Ⅱ) on 4A zeolite synthesized by aluminum extraction residue by fly ash [J]. Inorganic Chemicals Industry, 2024, 56(10): 127-134. |
| [10] | XU Li, ZHANG Qiang. Experimental study on properties of iron tailings powder cement-based materials [J]. Inorganic Chemicals Industry, 2023, 55(6): 116-123. |
| [11] | LI Wen, WANG Wenxiang, FANG Hongsheng, WU Pingxiao. Study on effect mechanism of silicon-aluminum additives on stabilization of heavy metals in fly ash by mechanochemical stabilization method [J]. Inorganic Chemicals Industry, 2023, 55(4): 84-91. |
| [12] | SUN Zhigao, WU Yuan, YANG Xingchun, ZHANG Dongliang, WANG Mitang. Preparation and properties of high-magnesium nickel slag-fly ash based geopolymer [J]. Inorganic Chemicals Industry, 2023, 55(11): 139-146. |
| [13] | YANG Hongjun, WANG Min, GE Haiwen, QIAO Youmin, QIAO Ziyang. Study on recycling process of potassium from calcium aluminate fly ash [J]. Inorganic Chemicals Industry, 2023, 55(10): 121-127. |
| [14] | HE Wenchao,XUE Jing,WANG Wei. Research on strength and creep characteristics of concrete containing fly ash microbead [J]. Inorganic Chemicals Industry, 2023, 55(1): 124-128. |
| [15] | LIU Darui,XU Lijun,LI Shichun,CAO Kun,TU Ya,LI Wenqing,LIU Qingliang. Research progress of recovery of strategic metal lithium from fly ash [J]. Inorganic Chemicals Industry, 2023, 55(1): 56-63. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||