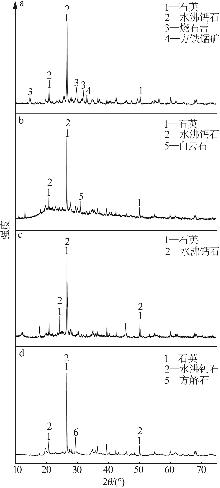
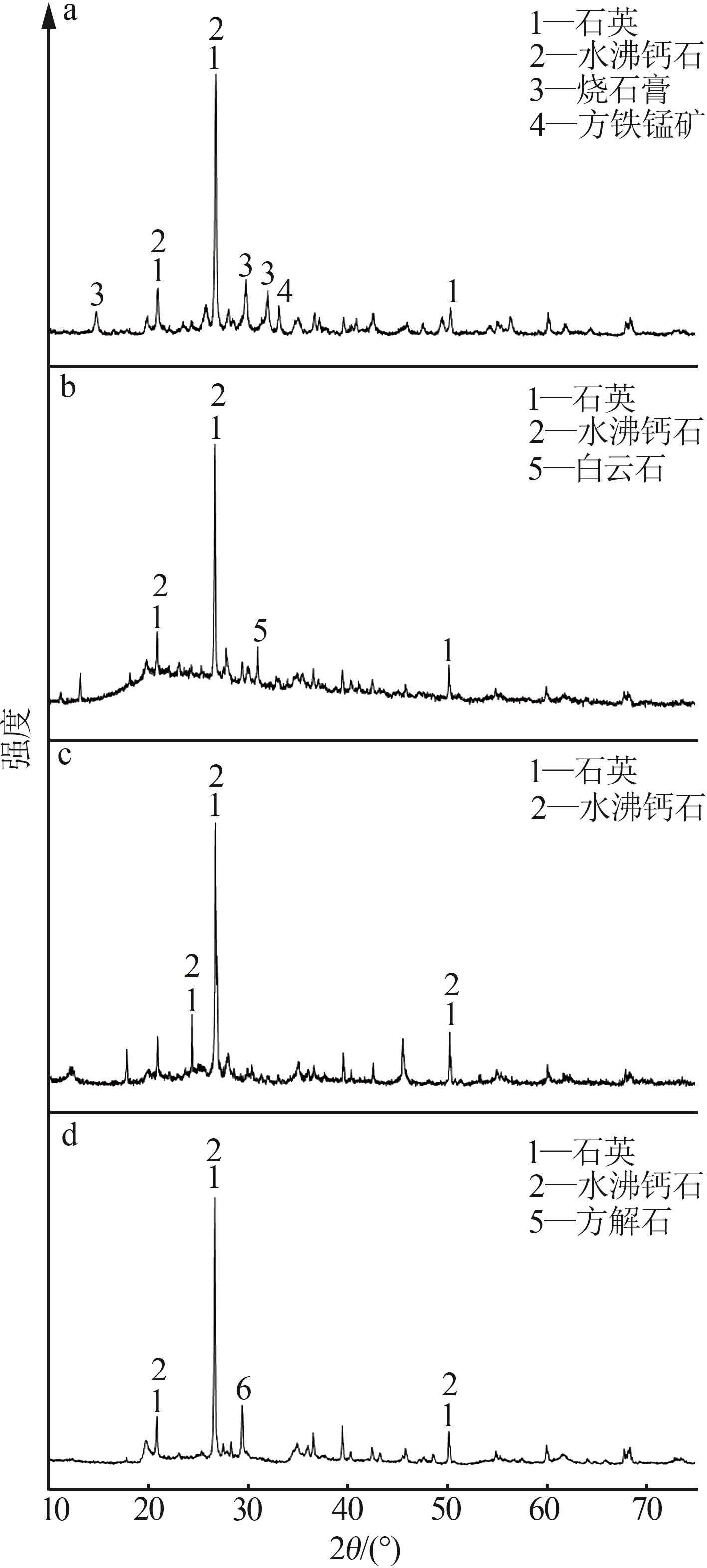
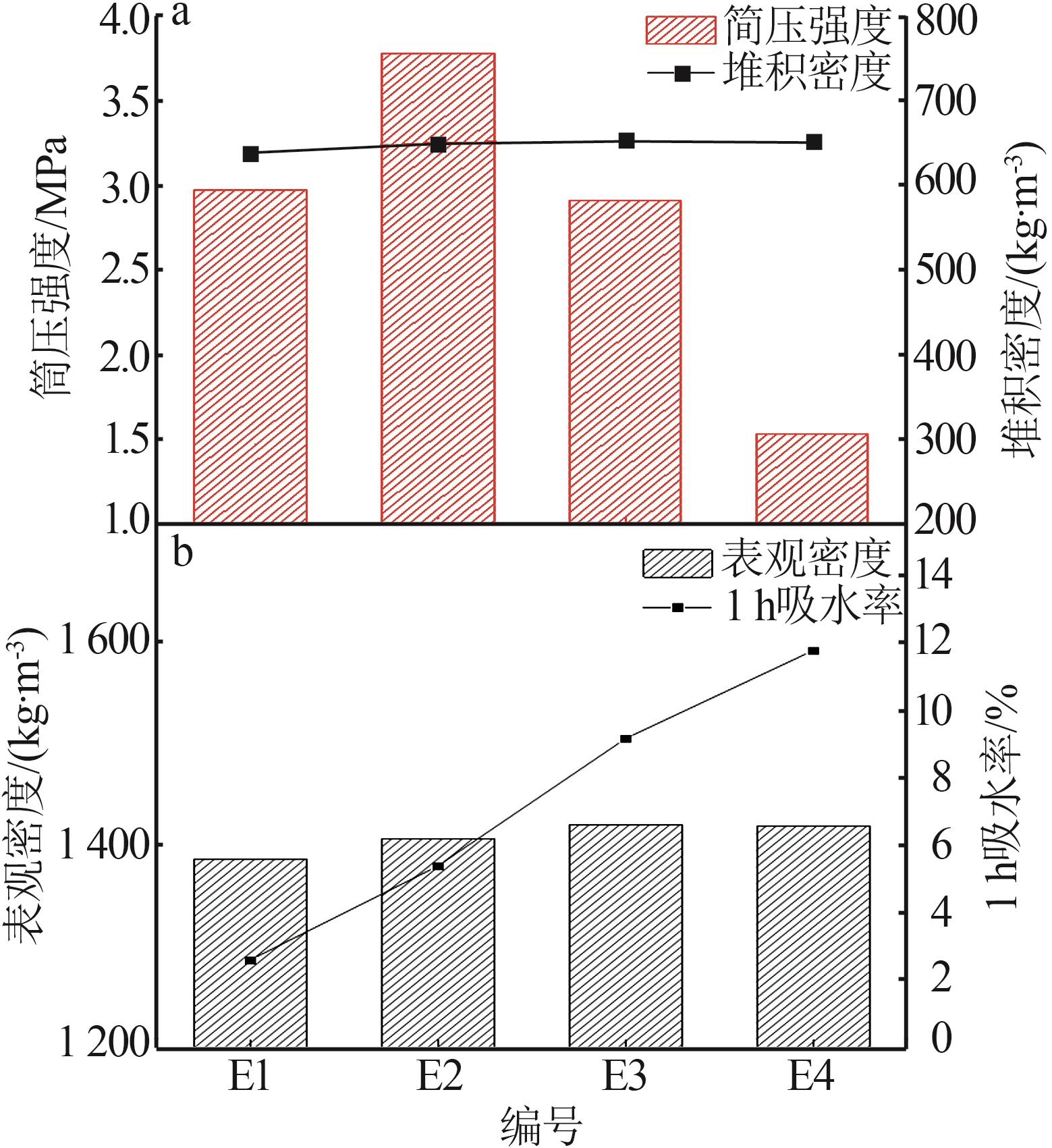
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (8): 102-109.doi: 10.19964/j.issn.1006-4990.2024-0491
• Environment·Health·Safety • Previous Articles Next Articles
Study on mechanism of preparation of porous ceramsite and fixation of heavy metal manganese from electrolytic manganese slag and multi-source solid waste
LIANG Qingyang1,2,3( ), XUE Fei1,2,3, HUANG Xuquan1,2,3(
), XUE Fei1,2,3, HUANG Xuquan1,2,3( ), ZHAO Xiaorong1,2,3, WANG Haojie1,2,3, HAN Zilong1,2,3, WANG Jun1,2,3
), ZHAO Xiaorong1,2,3, WANG Haojie1,2,3, HAN Zilong1,2,3, WANG Jun1,2,3
- 1.Engineering Research Center of Eco-environment in Three Gorges Reservoir Region,Ministry of Education,China Three Gorges University,Yichang 443002,China
2.College of Hydraulic & Environmental Engineering,China Three Gorges University Yichang 443002,China
3.Yichang Key Laboratory of Solid Waste Disposal and Resource Utilization(China Three Gorges University),Yichang 443002,China
-
Received:2024-09-10Online:2025-08-10Published:2024-11-25 -
Contact:HUANG Xuquan E-mail:LiangQY0605@163.com;huangxuquan@126.com
CLC Number:
Cite this article
LIANG Qingyang, XUE Fei, HUANG Xuquan, ZHAO Xiaorong, WANG Haojie, HAN Zilong, WANG Jun. Study on mechanism of preparation of porous ceramsite and fixation of heavy metal manganese from electrolytic manganese slag and multi-source solid waste[J]. Inorganic Chemicals Industry, 2025, 57(8): 102-109.
share this article
| [1] | HE Shichao, JIANG Daoyan, HONG Minghao,et al.Hazard-free treatment and resource utilisation of electrolytic manganese residue:A review[J].Journal of Cleaner Production,2021,306:127224. |
| [2] | WEN Quan, LIU Bo, ZHANG Junjie,et al.Efficient leaching of manganese from electrolytic manganese residue and recovery of the leaching solution:Reuse and purification[J].Separation and Purification Technology,2024,332:125648. |
| [3] | HE Weilong, LI Rui, ZHANG Yu,et al.Synergistic use of electrolytic manganese residue and barium slag to prepare belite-sulphoaluminate cement study[J].Construction and Building Materials,2022,326:126672. |
| [4] | HE Dejun, CHEN Mengjun, LIU Hui.Electrolytic manganese residue pretreated by filter press-water washing and carbide slag:Manganese recycling,depth harmless and dispersion[J].Process Safety and Environmental Protection,2024,186:1297-1306. |
| [5] | 黄川,王飞,谭文发,等.电解锰渣烧制陶粒的试验研究[J].非金属矿,2013,36(5):11-13. |
| HUANG Chuan, WANG Fei, TAN Wenfa,et al.Experimental study of sintered electrolytic manganese residue ceramic granules[J].Non-Metallic Mines,2013,36(5):11-13. | |
| [6] | 胡超超,王里奥,詹欣源,等.城市生活垃圾焚烧飞灰与电解锰渣烧制陶粒[J].环境工程学报,2019,13(1):177-185. |
| HU Chaochao, WANG Li′ao, ZHAN Xinyuan,et al.Preparation of ceramsite with MSWI fly ash and electrolytic manganese residues[J].Chinese Journal of Environmental Engineering,2019,13(1):177-185. | |
| [7] | 叶东东,徐子芳,赵怡梵,等.电解锰渣陶粒共烧结温度影响机理研究[J].材料导报,2022,36(11):89-94. |
| YE Dongdong, XU Zifang, ZHAO Yifan,et al.Influence mechanism of EMR ceramsite co-sintering temperature[J].Materials Reports,2022,36(11):89-94. | |
| [8] | KE Xuan, CHEN Xiaohong, WANG Ting,et al.Immobilization mechanism of Mn2+ in electrolytic manganese residue using sintered ceramsites prepared by alkali-mechanical-roasting method[J].Journal of Environmental Chemical Engineering,2024,12(2):112419. |
| [9] | 张云昊,柴轶凡,安胜利,等.选冶固废制备陶粒技术研究现状及展望[J].金属矿山,2022(7):259-266. |
| ZHANG Yunhao, CHAI Yifan, AN Shengli,et al.Research status and prospects of ceramsite preparation technology using selecting and smelting solid waste[J].Metal Mine,2022(7):259-266. | |
| [10] | LONG Yuyang, PU Kai, YANG Yuqiang,et al.Preparation of High-strength ceramsite from municipal solid waste incineration fly ash and clay based on CaO-SiO2-Al2O3 system[J].Construction and Building Materials,2023,368:130492. |
| [11] | PEI Jiannan, PAN Xiaolin, QI Yafei,et al.Preparation and characterization of ultra-lightweight ceramsite using non-expanded clay and waste sawdust[J].Construction and Building Materials,2022,346:128410. |
| [12] | 高育欣,李坤杰,马建峰,等.城市污泥掺量对煤气化渣陶粒性能影响[J].硅酸盐通报,2021,40(2):579-586. |
| GAO Yuxin, LI Kunjie, MA Jianfeng,et al.Influence of municipal sludge dosage on performance of coal gasification slag ceramsite[J].Bulletin of the Chinese Ceramic Society,2021,40(2):579-586. | |
| [13] | 罗晖,钱觉时,陈伟,等.污水污泥页岩陶粒烧胀特性[J].硅酸盐学报,2010,38(7):1247-1252. |
| LUO Hui, QIAN Jueshi, CHEN Wei,et al.Calcining expansion behavior of sewage sludge with shale[J].Journal of the Chinese Ceramic Society,2010,38(7):1247-1252. | |
| [14] | 王燕琳,贾冠华,杨凤玲.固硫灰-污泥陶粒的制备及其性能影响研究[J].非金属矿,2022,45(3):98-102,106. |
| WANG Yanlin, JIA Guanhua, YANG Fengling.Study on preparation and properties of CFBC fly ash-sludge ceramsite[J].Non-Metallic Mines,2022,45(3):98-102,106. | |
| [15] | 李煜,赵伟,张兴华,等.高强萤石尾矿陶粒的制备及其性能研究[J].无机盐工业,2023,55(5):100-108,136. |
| LI Yu, ZHAO Wei, ZHANG Xinghua,et al.Syudy on preparation of high-strength ceramsite from fluorite tailings and its properti- | |
| es[J].Inorganic Chemicals Industry,2023,55(5):100-108,136. | |
| [16] | JIANG Jun, CHEN Song, JIN Chao,et al.Preparation and properties of high-strength lightweight aggregate ceramsite from nepheline tailings[J].Construction and Building Materials,2023,368:130458. |
| [17] | 刘明伟,许国仁,李圭白.Fe2O3对污泥与底泥制备轻质陶粒性能的影响[J].哈尔滨工业大学学报,2012,44(10):18- 21. |
| LIU Mingwei, XU Guoren, LI Guibai.Effect of Fe2O3 on the characteristics of lightweight aggregate made from sewage sludge and river sediment[J].Journal of Harbin Institute of Technology,2012,44(10):18-21. | |
| [18] | 罗树琼,江壮壮,张海波,等.微波技术烧成拜耳法赤泥-渣土陶粒[J].非金属矿,2022,45(4):97-101,106. |
| LUO Shuqiong, JIANG Zhuangzhuang, ZHANG Haibo,et al.Microwave technology sintering bayer red mud-muck ceramsites[J].Non-Metallic Mines,2022,45(4):97-101,106. | |
| [19] | 王志明,孙玉宁,王永龙,等.XRD和FTIR的掺硅酸钠富水材料强度演化机制[J].光谱学与光谱分析,2019,39(10): 3199. |
| WANG Zhiming, SUN Yuning, WANG Yonglong,et al.Strength evolution mechanism of water-rich material blending with sodium silicate by XRD and FTIR[J].Spectroscopy and Spectral Analysis,2019,39(10):3199. | |
| [20] | WANG Chaoqiang, DUAN Dingyi, HUANG Deming,et al.Lightweight ceramsite made of recycled waste coal gangue & municipal sludge:Particular heavy metals,physical performance and human health[J].Journal of Cleaner Production,2022,376:134309. |
| [21] | XU Wei, ZHANG Yuyue, YAN Jun,et al.Microstructure and properties of high-strength lightweight ceramsites customised with ultra-fine copper tailings[J].Construction and Building Materials,2024,429:136433. |
| [22] | PEI Jiannan, PAN Xiaolin, LV Zhongyang,et al.Synergistic mechanism to prepare ultra-lightweight ceramsite using multiple industrial solid wastes[J].Construction and Building Materials,2024,425:136139. |
| [23] | 罗忠涛,肖宇领,杨久俊,等.垃圾焚烧飞灰有毒重金属固化稳定技术研究综述[J].环境污染与防治,2012,34(8):58-62,68. |
| LUO Zhongtao, XIAO Yuling, YANG Jiujun,et al.Progress on the solidification of toxic heavy metals in municipal solid waste incineration fly ash[J].Environmental Pollution & Control,2012,34(8):58-62,68. | |
| [24] | 柯国鹏,徐浩,杨如柱,等.典型固废基烧结陶粒工艺、重金属转化行为及其应用现状分析[J].硅酸盐通报,2024,43(8):2912-2923. |
| KE Guopeng, XU Hao, YANG Ruzhu,et al.Process,heavy metal transformation behavior of typical solid waste-based sintering ceramsite and its application status analysis[J].Bulletin of the Chinese Ceramic Society,2024,43(8):2912-2923. |
| [1] | ZHAO Xueqing, WANG Zhonghui, WANG Yongwei, WANG Zhiyu, HUO Xiaodong. Study on preparation of nitrogen-doped porous carbon materials derived from used cigarette filters and their adsorption properties [J]. Inorganic Chemicals Industry, 2025, 57(7): 110-119. |
| [2] | ZHANG Li, HU Qingxun, CHEN Jun, LIU Huangfei, FANG Hua, WANG Jiujiang, LIU Honghai, CAO Gengzhen. Study on highly active FCC matrix materials prepared by suspended solids and catalytic performance [J]. Inorganic Chemicals Industry, 2025, 57(7): 127-133. |
| [3] | DANG Shimin, GUO Jianxia, LI Haichao. Study on preparation of activated carbon of Stellera chamaejasme Linn by cesium chloride chemical method [J]. Inorganic Chemicals Industry, 2025, 57(6): 64-69. |
| [4] | CHAI Chunjing, FENG Zhengjun, WU Haibin, SHI Xiaokai, ZHANG Junjie, SONG Huiping. Effect of coal gangue-based porous matrix on soil solute transport [J]. Inorganic Chemicals Industry, 2024, 56(9): 107-116. |
| [5] | SUN Yanlong, YUAN Guangsheng, WANG Hongjun. Study on palladium modified mesoporous TiO2 nanorods for efficient photodegradation of tetracycline [J]. Inorganic Chemicals Industry, 2024, 56(9): 147-153. |
| [6] | WANG Wei, LI Wei, LI Limin, LIU Dongxu. Study on pretreatment of iron phosphate production wastewater reuse and zero discharge [J]. Inorganic Chemicals Industry, 2024, 56(8): 99-103. |
| [7] | LIU Qingcui, LI Yunqing, PANG Ruiqi, TIAN Yaping, CHEN Yiying, LI Fang, LI Qiming. Preparation of Zn/Co-ZIF derived porous carbon supported Pd as catalyst and its application to formic acid dehydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(6): 147-152. |
| [8] | LI Jiangpeng, ZHANG Huibin. Synergistic degradation of methylene blue by photo-Fenton and photocatalytic with 3D porous LaFeO3/CeO2/SrTiO3 [J]. Inorganic Chemicals Industry, 2024, 56(5): 141-148. |
| [9] | ZHAO Tianting, ZHU Delun, YANG Lin, ZHOU Xinlei. Preparation and process optimization of porous silicon anode materials for lithium-ion battery [J]. Inorganic Chemicals Industry, 2024, 56(5): 31-38. |
| [10] | TAO Longhai, WANG Yongjie, DUAN Jiatang, SHU Yizhou, WAN Banglong, HUANG Chengdong, ZHANG Yueqiang, LU Zhenya. Research progress and prospect of sludge acid and raffinate acid by-product of wet-process phosphoric acid [J]. Inorganic Chemicals Industry, 2024, 56(3): 12-18. |
| [11] | NI Dong, TANG Liang, HE Zhaoyi, WANG Jian, PEI Shanshan, XIA Lei. Study on sulfate activation performance of electrolytic manganese residue in hydrated lime-slag system [J]. Inorganic Chemicals Industry, 2024, 56(11): 151-157. |
| [12] | CHEN Yanmeng, MO Huiling, ZHONG Jiamei, YANG Pengfei, LAN Junfeng. Study on preparation process of porous calcium carbonate by carbonization [J]. Inorganic Chemicals Industry, 2023, 55(8): 102-108. |
| [13] | TIAN Yuling, CHENG Yang, HAN Rong, ZHOU Mei, WANG Chengjie, GE Qiangru. Effect of CaO on heavy metals stability and adsorption properties of sludge-derived biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 124-129. |
| [14] | LU Dachuan, GU Weihua, ZHAO Jing, ZHUANG Xuning, DONG Bin, BAI Jianfeng. Research progress of influencing factors of pyrolysis of municipal sludge and residue recycling [J]. Inorganic Chemicals Industry, 2023, 55(5): 8-15. |
| [15] | DONG Xinyu, WANG Haifeng, HE Yue, YANG Pan, WANG Song, YANG Chunyuan, WANG Qin, HUANG Bifang, WANG Jiawei. Exleaching toxicity analysis and harmless treatment of electrolytic manganese residue [J]. Inorganic Chemicals Industry, 2023, 55(5): 85-90. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||