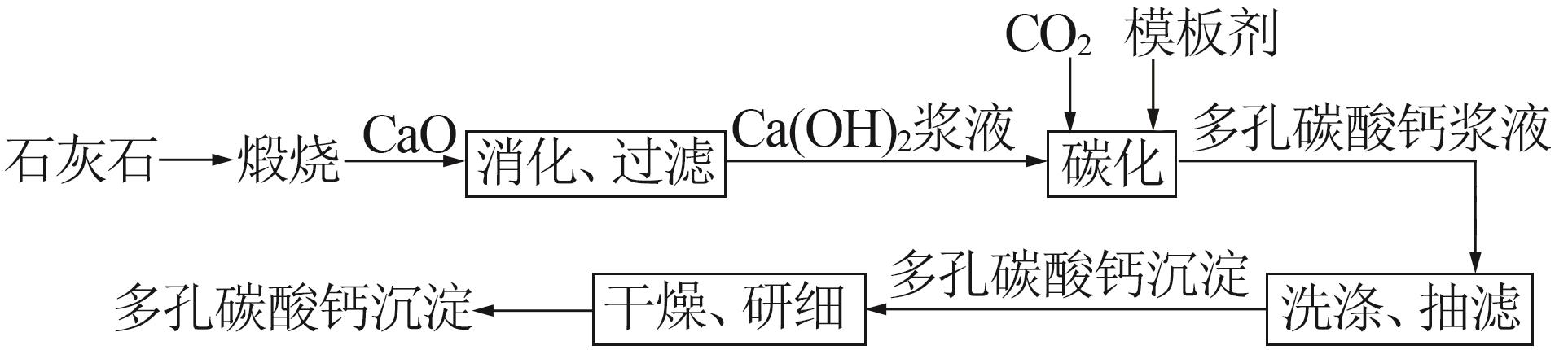
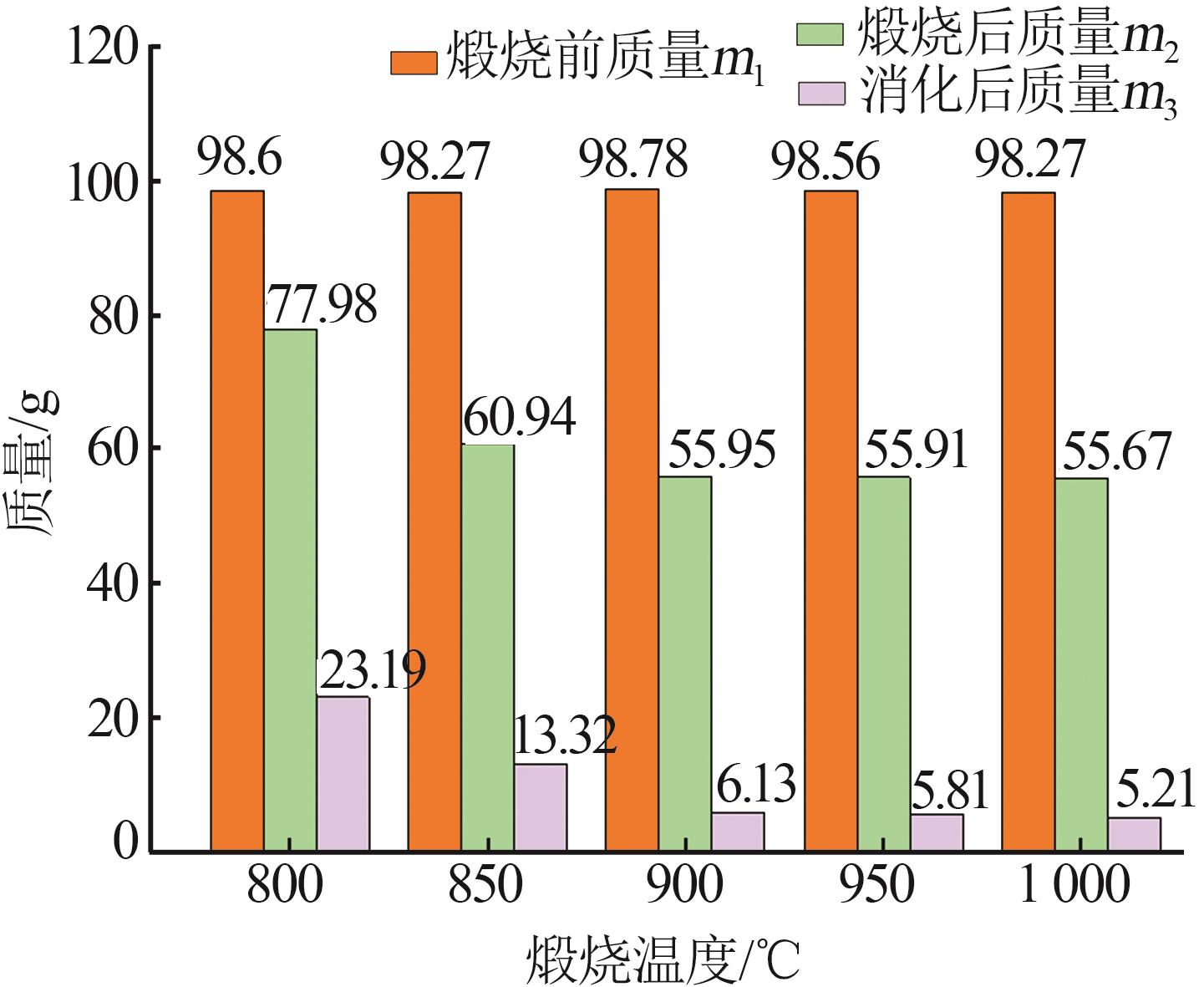
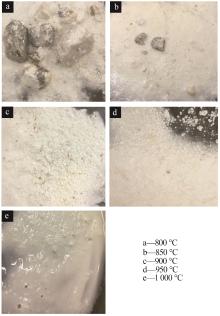
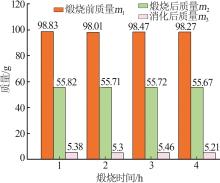
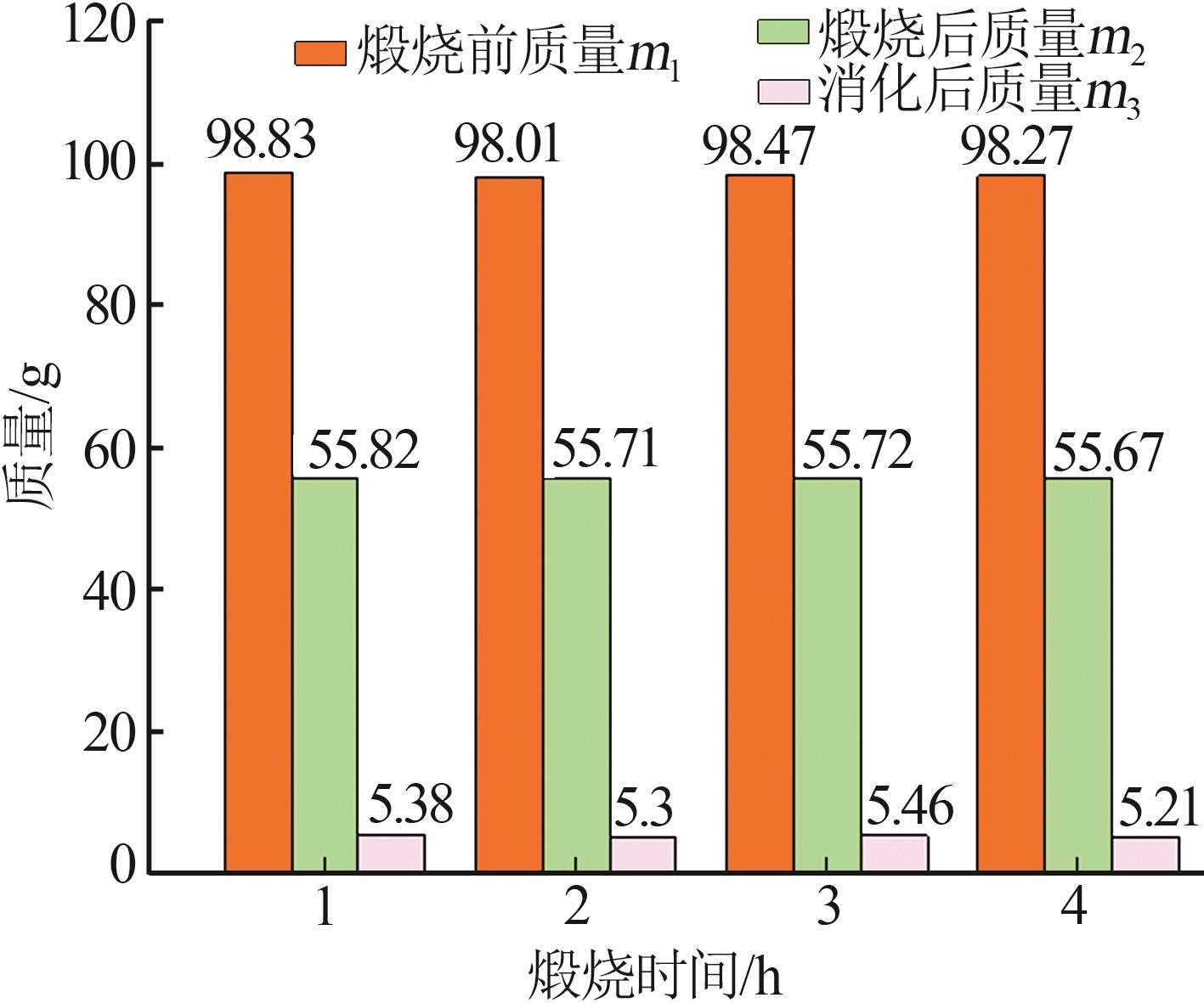
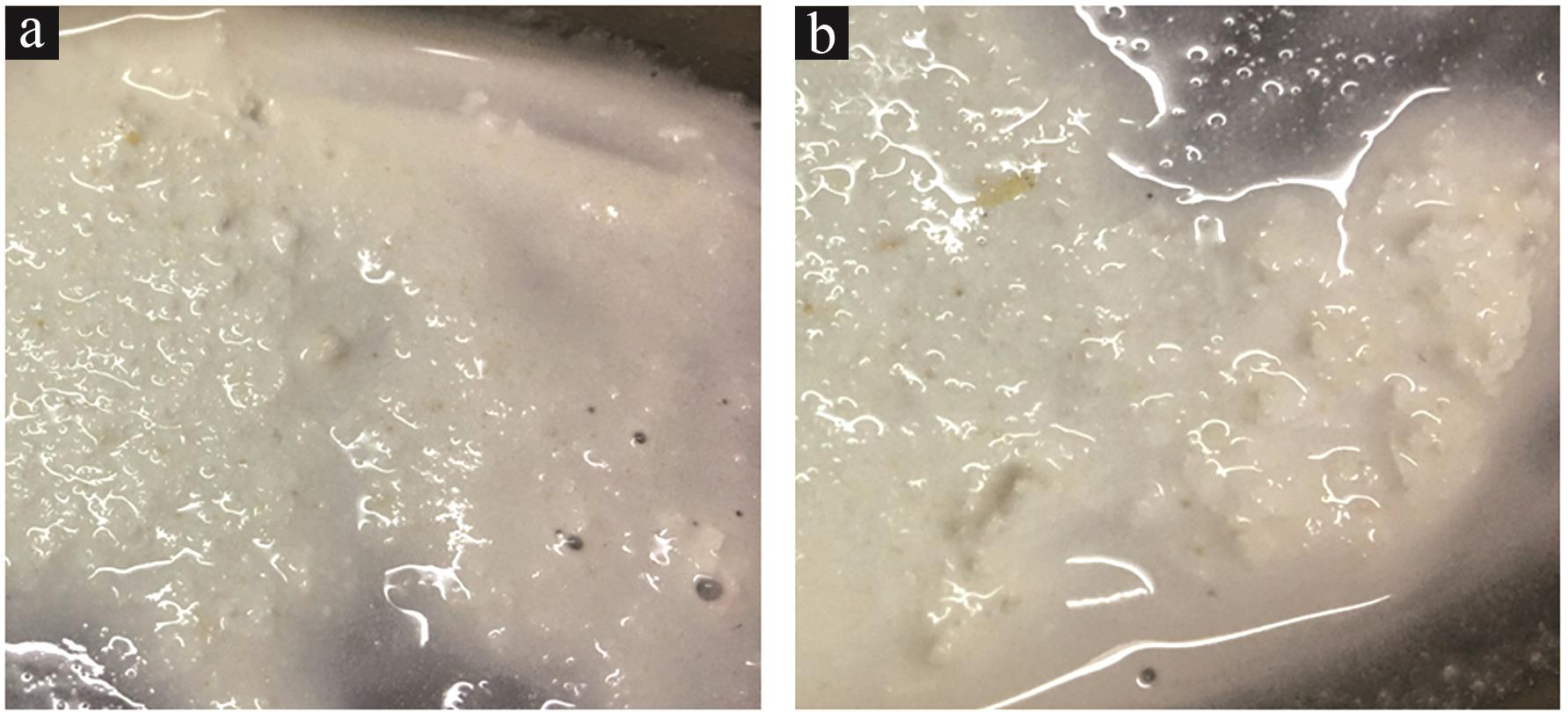
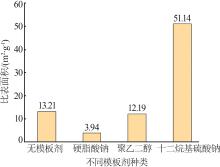
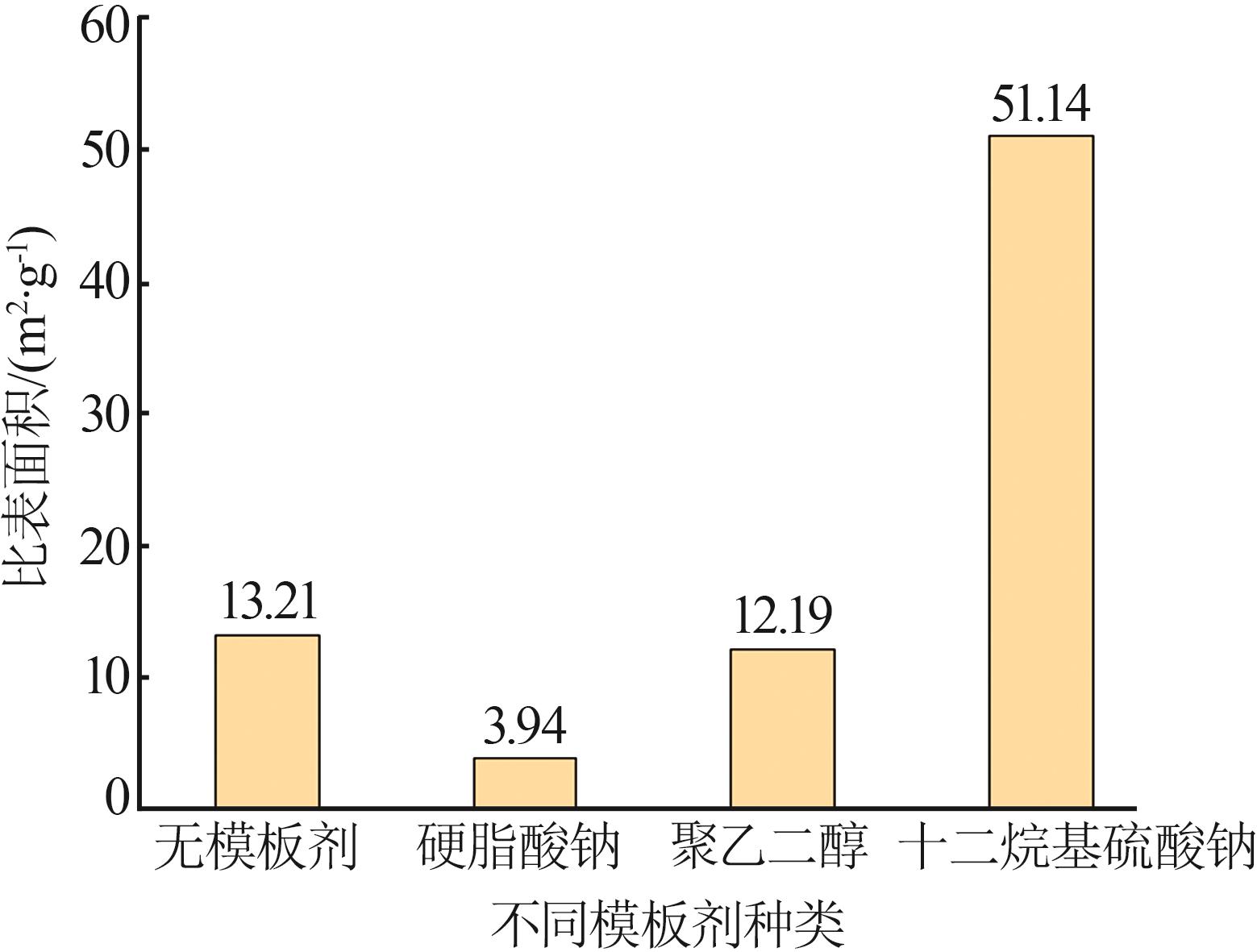
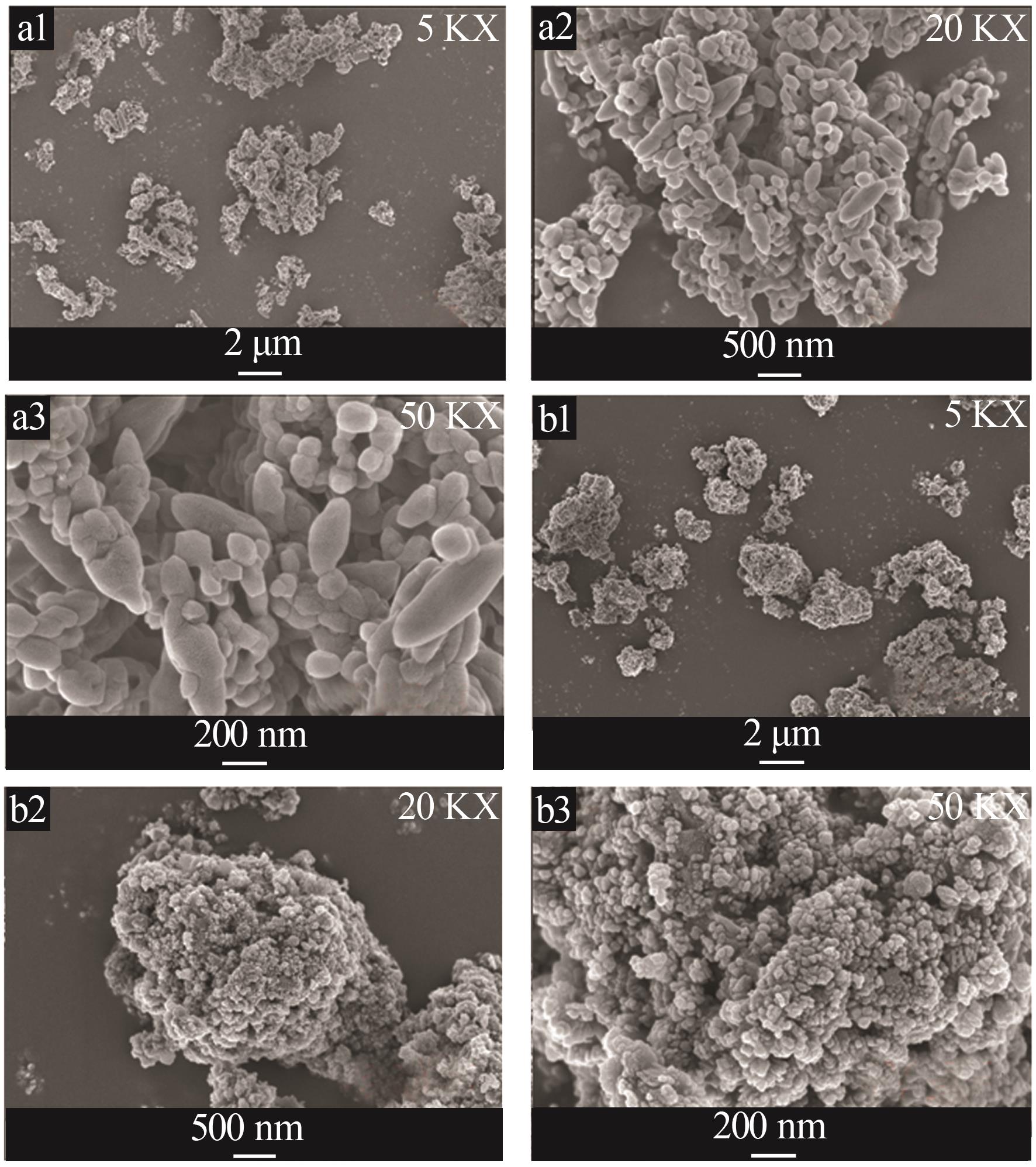
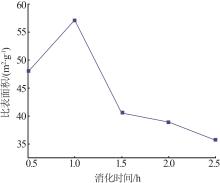
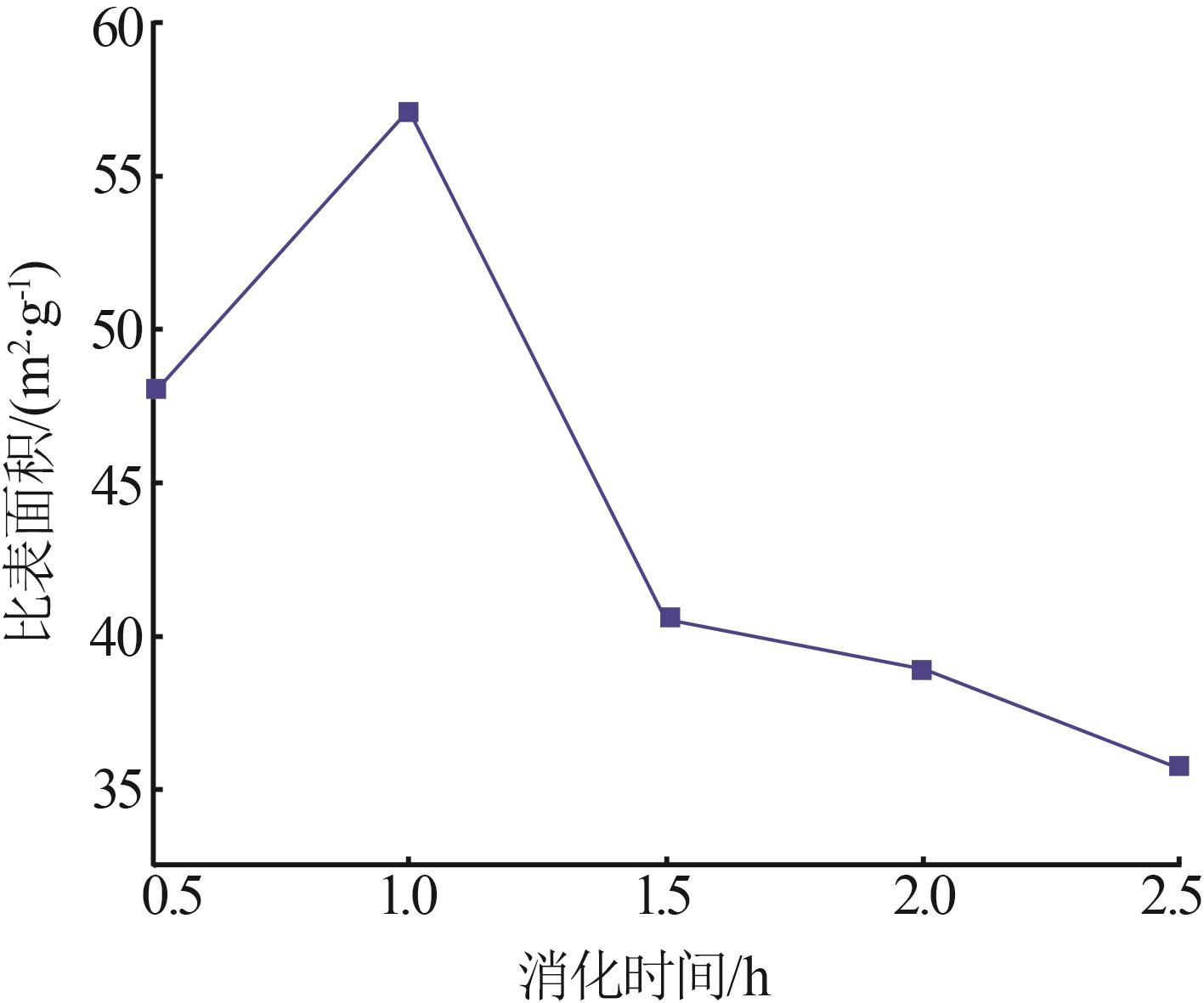
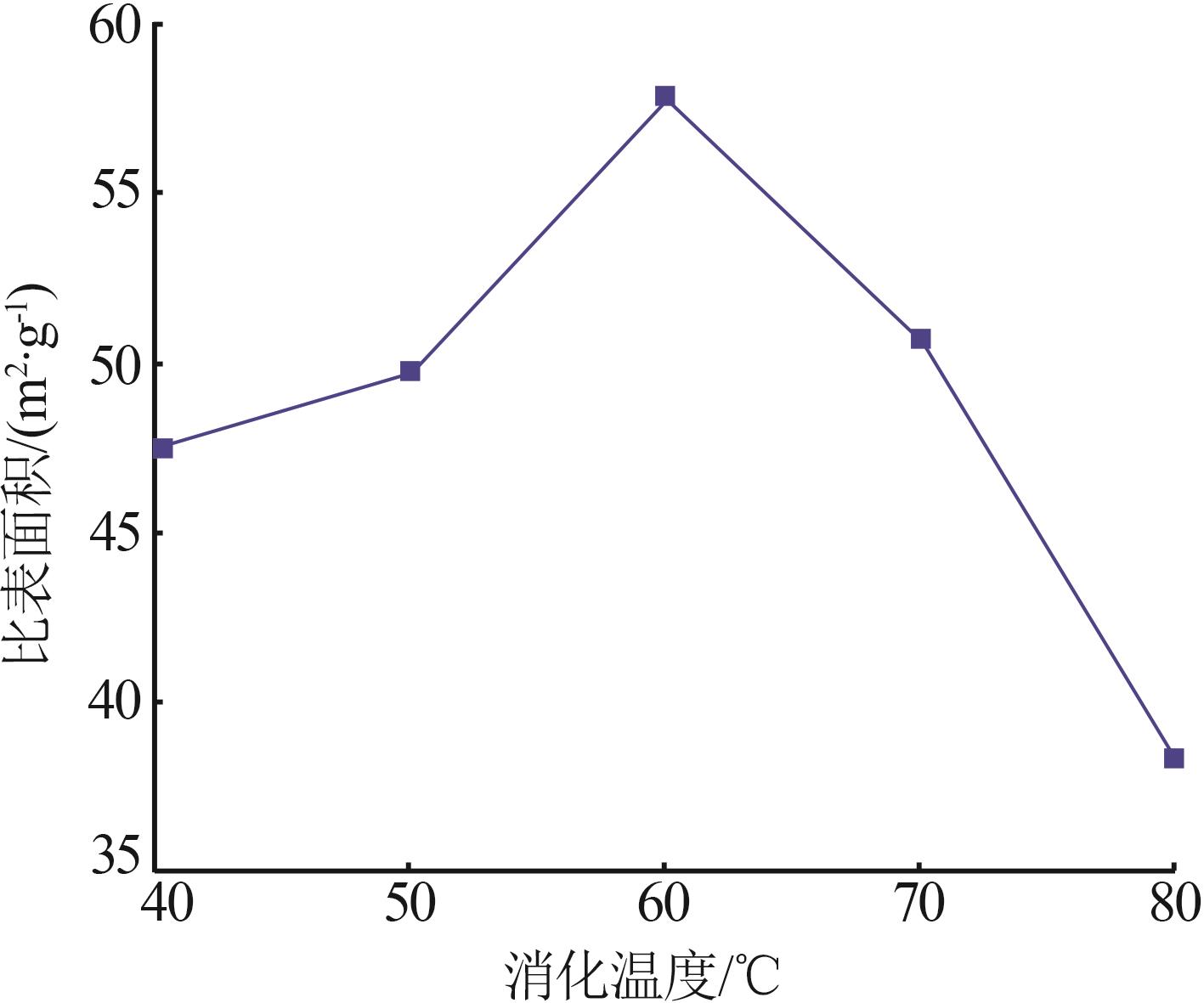
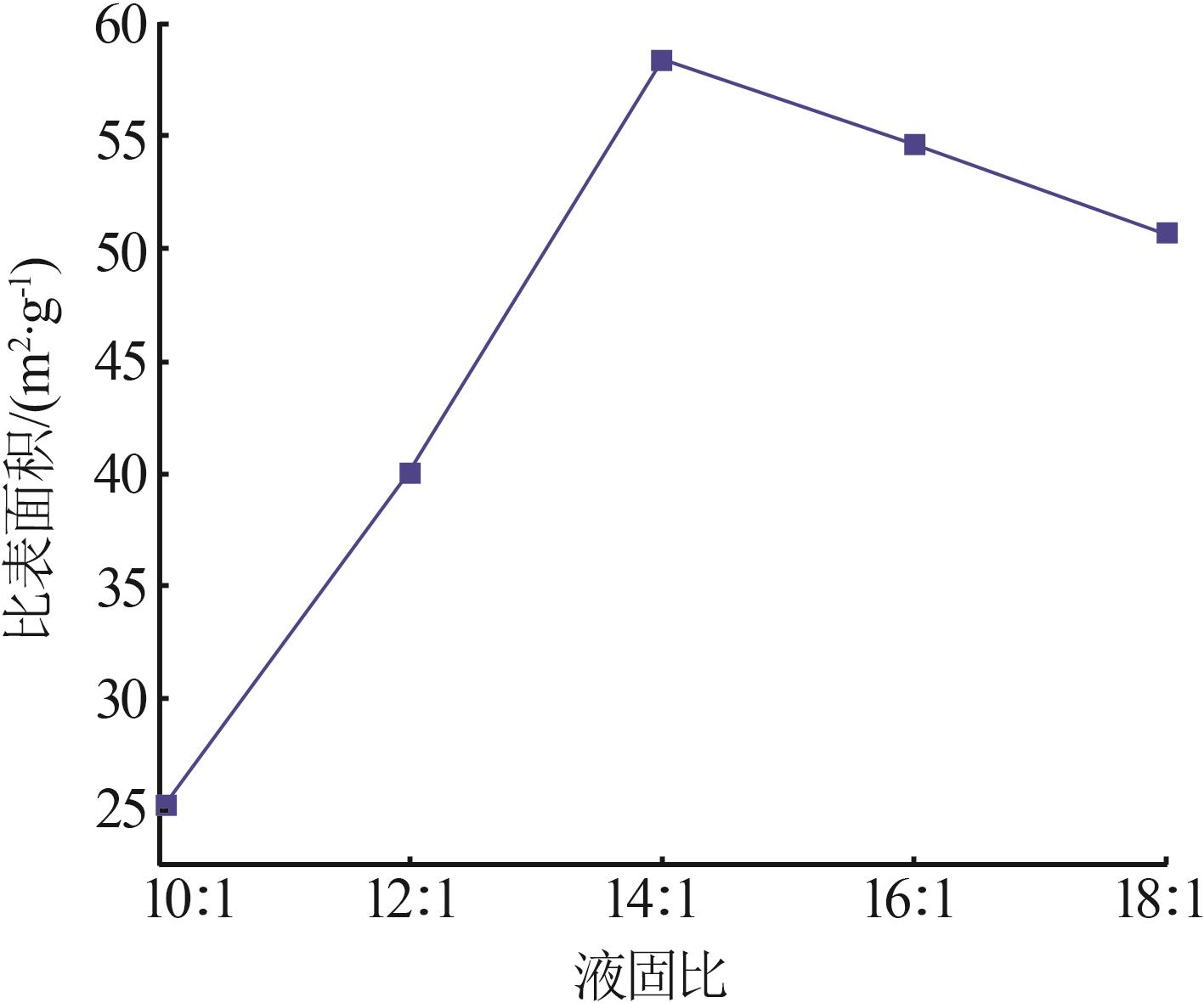
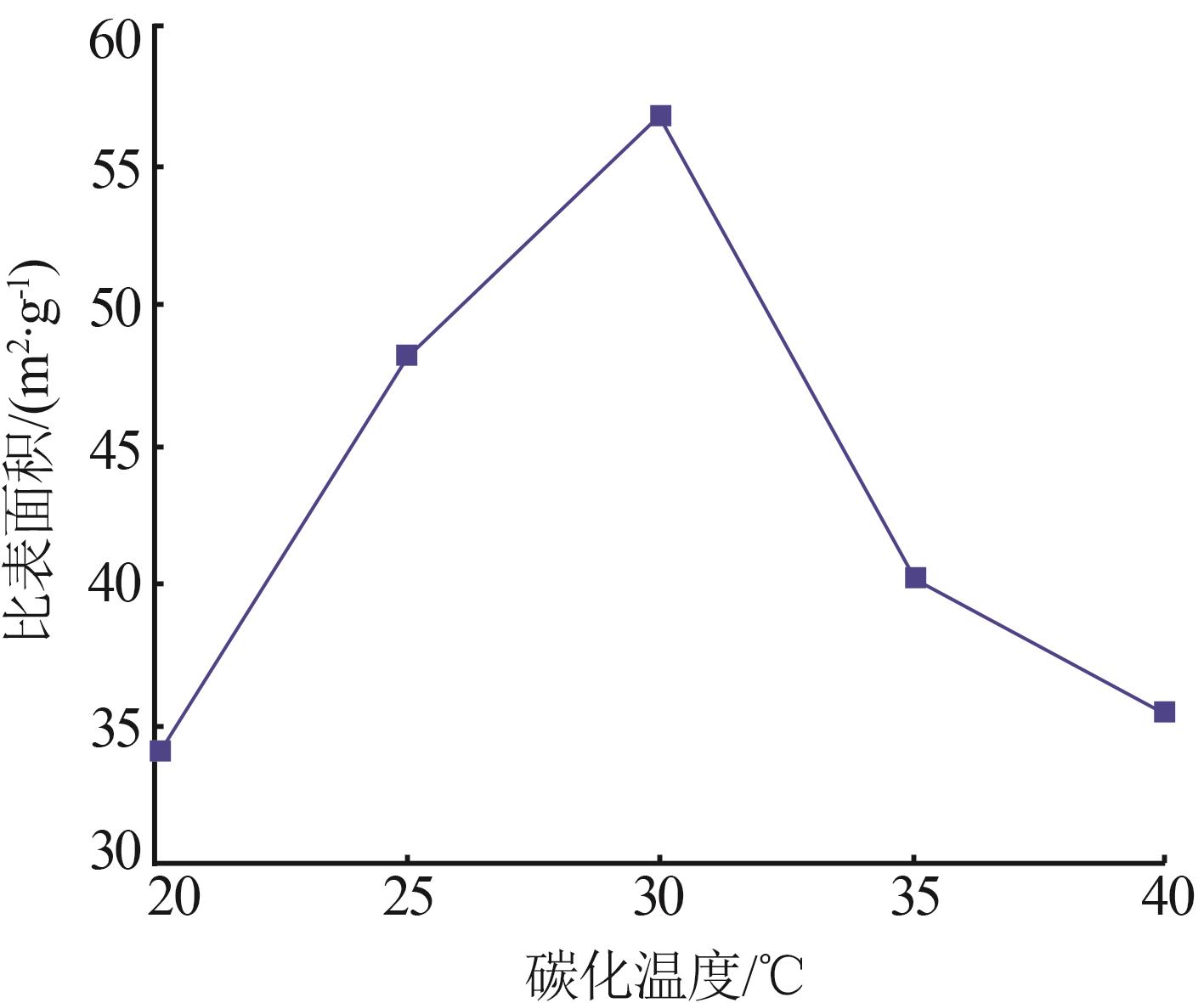
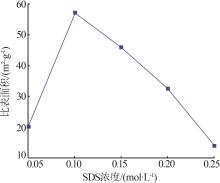
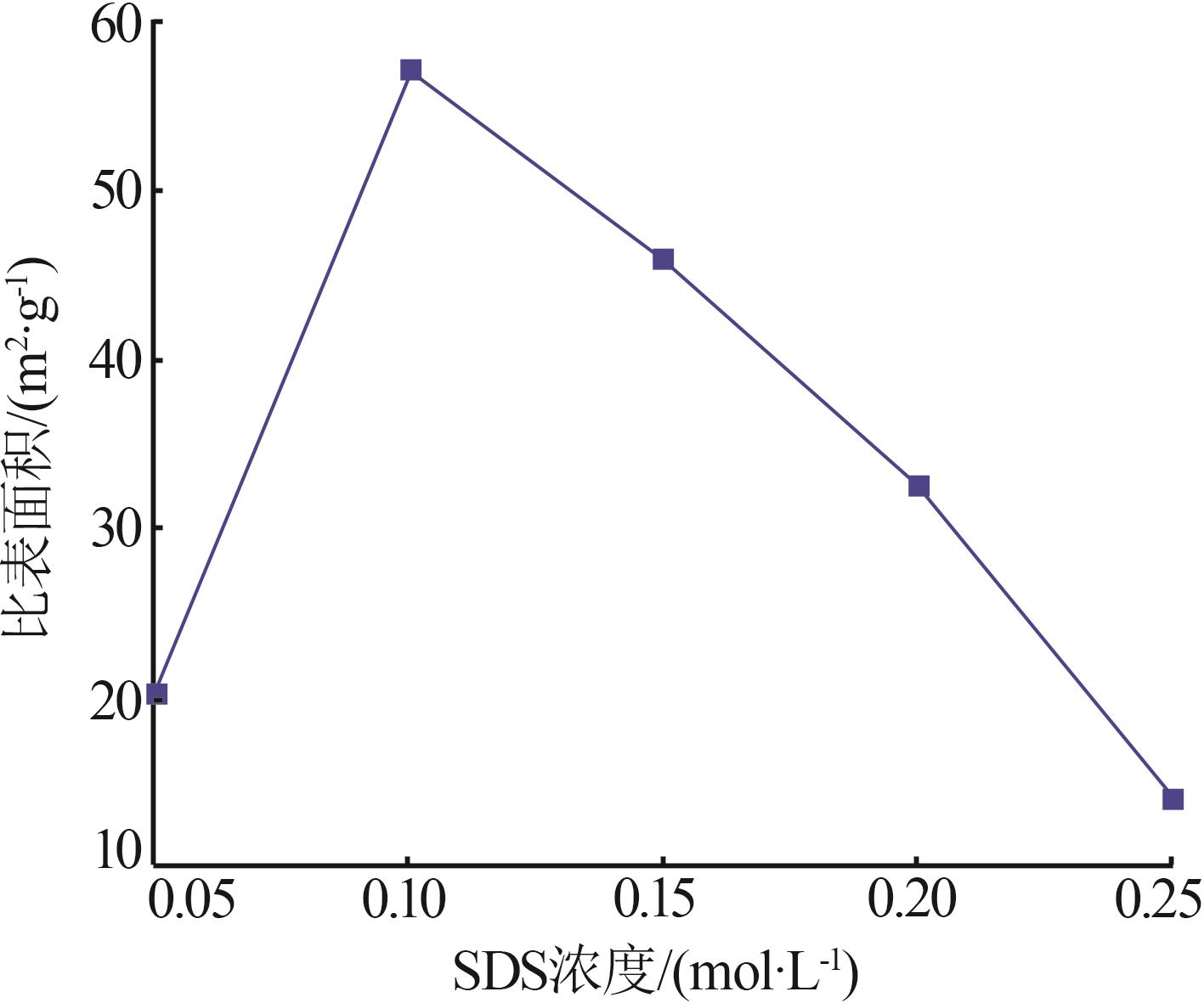
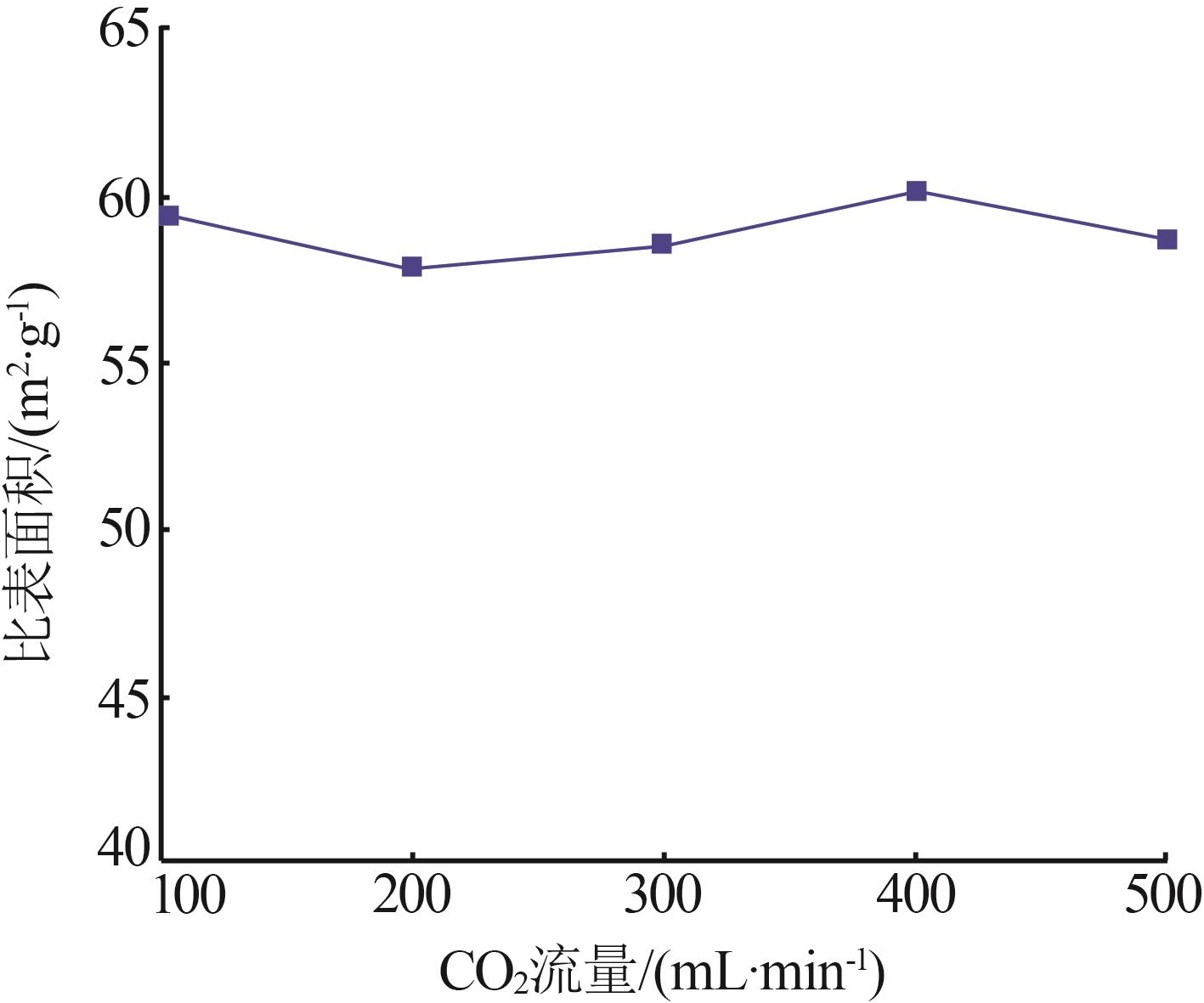
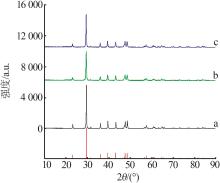
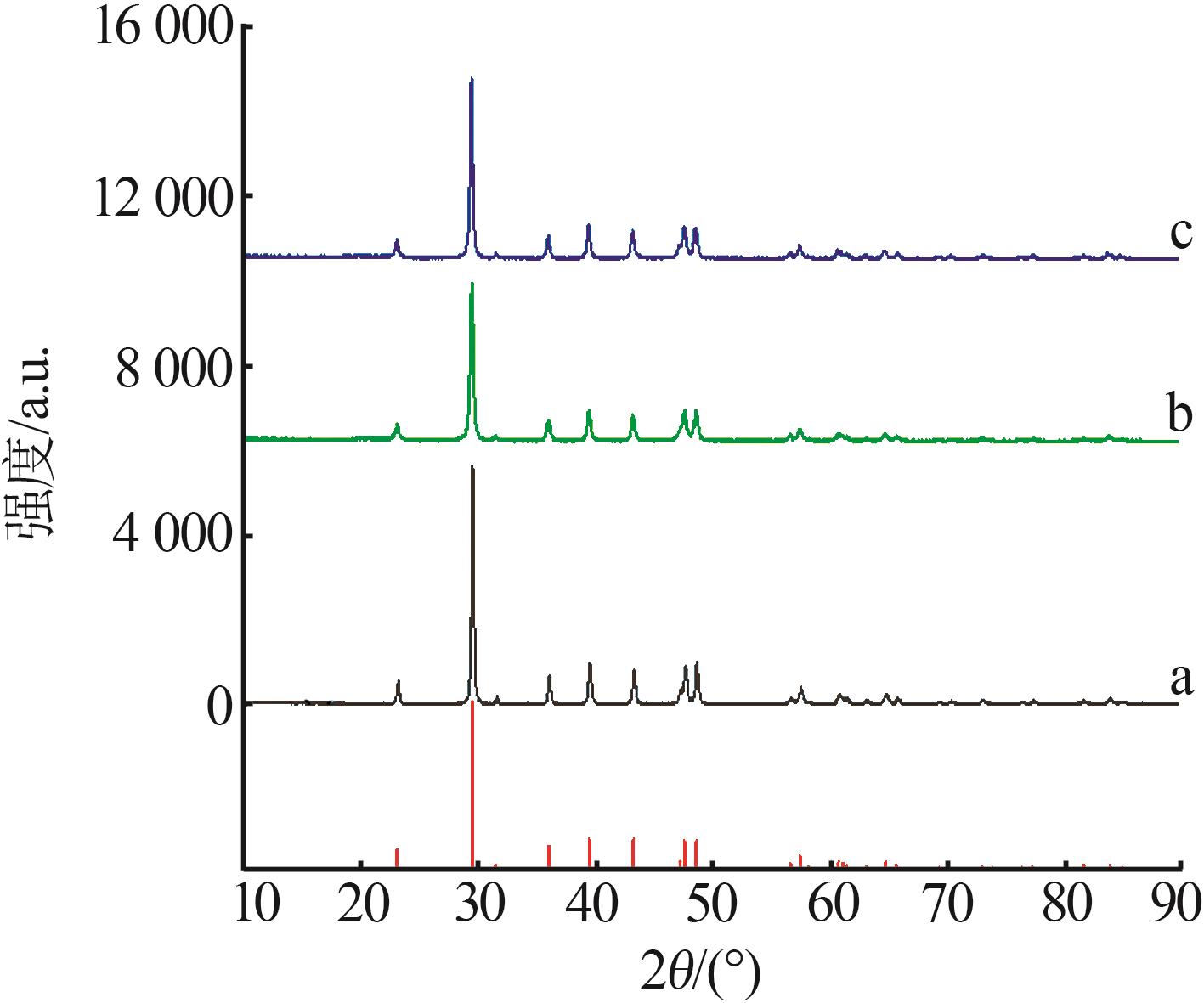
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (8): 102-108.doi: 10.19964/j.issn.1006-4990.2022-0594
• Research & Development • Previous Articles Next Articles
Study on preparation process of porous calcium carbonate by carbonization
CHEN Yanmeng( ), MO Huiling, ZHONG Jiamei, YANG Pengfei, LAN Junfeng(
), MO Huiling, ZHONG Jiamei, YANG Pengfei, LAN Junfeng( )
)
- College of Food & Biochemical Engineering,Guangxi Science & Technology Normal University,Laibin 546199,China
-
Received:2022-10-08Online:2023-08-10Published:2023-08-25 -
Contact:LAN Junfeng E-mail:277153429@qq.com;272639515@qq.com
CLC Number:
Cite this article
CHEN Yanmeng, MO Huiling, ZHONG Jiamei, YANG Pengfei, LAN Junfeng. Study on preparation process of porous calcium carbonate by carbonization[J]. Inorganic Chemicals Industry, 2023, 55(8): 102-108.
share this article
| 1 | 闫静.纳米碳酸钙的制备条件优化及碳化结晶过程研究[D].杭州:浙江工业大学,2013. |
| YAN Jing.Study on preparation conditions of nano-CaCO3 and the carbonization-crystallization process[D].Hangzhou:Zhejiang University of Technology,2013. | |
| 2 | 周绿山,赖川,王芬,等.多孔碳酸钙的制备及应用研究进展[J].化工进展,2018,37(1):159-167. |
| ZHOU Lüshan, LAI Chuan, WANG Fen,et al.Progress in fabrication and applications of porous calcium carbonate[J].Chemical Industry and Engineering Progress,2018,37(1):159-167. | |
| 3 | 刘琬,王芳,肖惠宁.多孔碳酸钙的研究现状[J].广东化工,2016,43(24):85-86. |
| LIU Wan, WANG Fang, XIAO Huining.The Current research status of porous calcium carbonate[J].Guangdong Chemical Industry,2016,43(24):85-86. | |
| 4 | 黄文艺,马蓝宇,程昊,等.多孔碳酸钙微球的快速制备[J].应用化工,2017,46(6):1078-1081. |
| HUANG Wenyi, MA Lanyu, CHENG Hao,et al.Rapid synthesis of porous calcium carbonate microspheres[J].Applied Chemical Industry,2017,46(6):1078-1081. | |
| 5 | 陈燕萌,何春妮,潘亭,等.鸡蛋壳基多孔碳酸钙对刚果红吸附特性研究[J].无机盐工业,2021,53(8):91-95. |
| CHEN Yanmeng, HE Chunni, PAN Ting,et al.Study on adsorption characteristics of multi-pore calcium carbonate made from egg shell powder for Congo red[J].Inorganic Chemicals Industry,2021,53(8):91-95. | |
| 6 | 赵历,卓民权,龚福忠,等.碳化法制备球霰石碳酸钙微球及形成机理[J].无机盐工业,2021,53(3):38-43. |
| ZHAO Li, ZHUO Minquan, GONG Fuzhong,et al.Synthesis of vaterite CaCO3 microspheres by carbonization method and its formation mechanism[J].Inorganic Chemicals Industry,2021,53(3):38-43. | |
| 7 | LI Li, YANG Yang, LV Yirui,et al.Porous calcite CaCO3 microspheres:Preparation,characterization and release behavior as do- |
| xorubicin carrier[J].Colloids and Surfaces B:Biointerfaces,2020,186:110720. | |
| 8 | DARSHANA SENARATHNA D D T T, NAMAL ABEYSOORIYA K H D, DUNUWEERA S P,et al.Removal of phosphate from aqueous solutions using chemically synthesized vaterite polymorph of porous calcium carbonate nanoparticles under optimized conditions[J].Journal of Nanomaterials,2020.Doi:10.1155/2020/3931910. |
| 9 | 胡彩霞,胡辰鑫,彭剑龙,等.以可溶性淀粉为晶型调控剂制备多孔球形碳酸钙[J].无机盐工业,2021,53(5):51-55. |
| HU Caixia, HU Chenxin, PENG Jianlong,et al.Preparation of porous spherical calcium carbonate with soluble starch as crystallization controller[J].Inorganic Chemicals Industry,2021,53(5):51-55. | |
| 10 | 王君,蔡杰慧,韦雄雄,等.CaCO3微球的制备及表征[J].高校化学工程学报,2019,33(1):199-206. |
| WANG Jun, CAI Jiehui, WEI Xiongxiong,et al.Preparation and characterization of CaCO3 microparticles[J].Journal of Chemical Engineering of Chinese Universities,2019,33(1):199-206. | |
| 11 | 刘晨民,刘曦曦,陈小鹏,等.超重力反应结晶碳化法制备球形碳酸钙[J].化工进展,2021,40(11):6323-6331. |
| LIU Chenmin, LIU Xixi, CHEN Xiaopeng,et al.Preparation of spherical calcium carbonate by high-gravity reaction crystallization carbonization[J].Chemical Industry and Engineering Progress,2021,40(11):6323-6331. | |
| 12 | 童张法,胡超,李立硕,等.间歇鼓泡碳化法制备立方形纳米碳酸钙工艺条件优化[J].广西科学,2015,22(1):53-59. |
| TONG Zhangfa, HU Chao, LI Lishuo,et al.Optimization of processing conditions for the preparation of cubic nano-sized calcium carbonate by intermittent bubbling carbonation[J].Guangxi Sciences,2015,22(1):53-59. | |
| 13 | 刘琬.多孔碳酸钙及以其为模板高分子吸附剂的制备[D].北京:华北电力大学,2017. |
| LIU Wan.Fabrication of porous calcium carbonate and its application as a template for the preparation of polymeric adsorbent[D].Beijing:North China Electric Power University,2017. | |
| 14 | 李梦维,赵昌明,程铁欣,等.十二烷基苯磺酸钠对碳酸钙结晶行为的影响[J].高等学校化学学报,2018,39(11):2380-2385. |
| LI Mengwei, ZHAO Changming, CHENG Tiexin,et al.Influence of sodium dodecyl benzene sulfonate on crystallization behavior of calcium carbonate[J].Chemical Journal of Chinese Universities,2018,39(11):2380-2385. | |
| 15 | 魏仁零.石灰干式消化过程及机理研究[D].武汉:武汉科技大学,2011. |
| WEI Renling.The research in dry quicklime slaking process and mechanism[D].Wuhan:Wuhan University of Science and Technology,2011. | |
| 16 | 刘旭.纳米CaCO3碳化法制备、表面改性及在水性乳液中的应用[D].南宁:广西大学,2016. |
| LIU Xu.Preparation and surface mdification of nano calcium carbonate by carbonization cethod and application in aqueous emulsion[D].Nanning:Guangxi University,2016. | |
| 17 | 严雯莉.氢氧化钙的制备工艺及其碳化产物性能研究[D].上海:华东理工大学,2021. |
| YAN Wenli.Study on the preparation of calcium hydroxide and its carbonized product performance[D].Shanghai:East China University of Science and Technology,2021. | |
| 18 | 彭玉娇.碳化法制备球形碳酸钙的研究[D].南宁:广西大学,2017. |
| PENG Yujiao.Preparation of spherical calcium carbonate by carbonation[D].Nanning:Guangxi University,2017. | |
| 19 | 史刘宾,唐名德,汤勇,等.高压碳化法可控制备微纳米分级结构中空棒状碳酸钙及其表征[J].化工进展,2020,39(11):4742-4748. |
| SHI Liubin, TANG Mingde, TANG Yong,et al.Preparation and characterization of micro-nano hierarchical hollow rod-like calcium carbonate by high pressure carbonization[J].Chemical Industry and Engineering Progress,2020,39(11):4742-4748. | |
| 20 | 苏承炎,孙富强.碳化法制备碳酸钙工艺条件的探讨[J].广州化工,2013,41(16):108-109,117. |
| SU Chengyan, SUN Fuqiang.Study on the conditions of process of carbonation[J].Guangzhou Chemical Industry,2013,41(16):108-109,117. | |
| 21 | 王明,丁杨,闫红旭,等.碳化法形貌可控制备碳酸钙的研究[J].无机盐工业,2018,50(12):33-36. |
| WANG Ming, DING Yang, YAN Hongxu,et al.Study on morphology-controllable synthesis of calcium carbonate in carbonization process[J].Inorganic Chemicals Industry,2018,50(12):33-36. | |
| 22 | 常越凡,张慧捷,王珊珊,等.不同粒度纳米碳酸钙的可控制备[J].无机盐工业,2020,52(12):29-33. |
| CHANG Yuefan, ZHANG Huijie, WANG Shanshan,et al.Controllable preparation of nano-calcium carbonate with different particle sizes[J].Inorganic Chemicals Industry,2020,52(12):29-33. | |
| 23 | 张腾龙,庞艺川,冯杰.多孔碳酸钙微粒的简易制备及其形貌控制[J].高校化学工程学报,2015,29(2):377-381. |
| ZHANG Tenglong, PANG Yichuan, FENG Jie.Facile synthesis and morphology control of porous CaCO3 microparticles[J].Journal of Chemical Engineering of Chinese Universities,2015,29(2):377-381. | |
| 24 | 向乐凯,李枫,赵宁,等.二氧化碳鼓泡碳化法制备碳酸钙的研究[J].无机盐工业,2016,48(8):46-51. |
| XIANG Lekai, LI Feng, ZHAO Ning,et al.Study on preparation of calcium carbonate by carbon dioxide bubbling carbonation[J].Inorganic Chemicals Industry,2016,48(8):46-51. |
| [1] | YAN Xin, LIU Hailu, LIU Baolin, LIU Yi, LIU Yanyang. Research on key technologies and mechanisms of green nano calcium carbonate production [J]. Inorganic Chemicals Industry, 2025, 57(1): 71-76. |
| [2] | WANG Rui, ZHAO Hui, ZHAO Yuhang. Comparative study on decarbonization of aluminum extraction residue from fly ash by roasting and flotation [J]. Inorganic Chemicals Industry, 2024, 56(9): 136-141. |
| [3] | ZHANG Yaqi, CHEN Xiping, SUN Ningning. Study on preparation process of cryolite from anode carbon slag by catalytic decarburization [J]. Inorganic Chemicals Industry, 2024, 56(3): 91-97. |
| [4] | ZHOU Zhaoan, LI Jun, LIU Xiaowen, ZHOU Aiqing, MAO Anzhang. Study on carbonization and purification process of high COD industrial waste salt [J]. Inorganic Chemicals Industry, 2023, 55(9): 100-105. |
| [5] | REN Teng, LI Shengdong, WANG Dexi, CHU Fuzhou, SHAO Lixin. Numerical simulation analysis of influence of jet position on mixing effect of carbonization reactor [J]. Inorganic Chemicals Industry, 2023, 55(7): 109-114. |
| [6] | QU Xiaoyuan, ZHENG Qiang, FAN Yuanyang, LIU Haili, DENG Xiaoyang, LI Xue. Study on preparation of large cube calcium carbonate by secondary carbonization of dolomite [J]. Inorganic Chemicals Industry, 2023, 55(7): 58-64. |
| [7] | DENG Weilin,HUANG Wenke,HUANG Chongqiu,ZENG Xingbin. Regulatory preparation of porous calcium carbonate by polyacrylic acid and polystyrene sulfonate [J]. Inorganic Chemicals Industry, 2023, 55(2): 67-72. |
| [8] | ZHANG Shaokang,ZHAO Hua,LIU Runjing. Study on macro?kinetics of carbonization reaction of ammonia alkali salt sludge [J]. Inorganic Chemicals Industry, 2022, 54(6): 120-124. |
| [9] | ZHANG Shaokang,ZHAO Hua,LIU Runjing. Study on macro?kinetics of carbonization reaction of ammonia alkali salt sludge [J]. Inorganic Chemicals Industry, 2022, 54(6): 120-124. |
| [10] | QIAO Kun,LÜ Zening,YANG Lijun,DU Xiaoze. Research progress on effect of inorganic additives on regeneration process of carbon capture by ammonia [J]. Inorganic Chemicals Industry, 2022, 54(10): 79-86. |
| [11] | Yang Wenzhen,Xiong Ping,Sun Xiuyun,Han Weiqing. Study on combustion characteristics of waste pharmaceutical Na2SO4 and impurity removal by low-temperature carbonization [J]. Inorganic Chemicals Industry, 2021, 53(9): 76-82. |
| [12] | Chen Yanmeng,He Chunni,Pan Ting,Wang Yongshi,Meng Moji,Lan Junfeng. Study on adsorption characteristics of multi-pore calcium carbonate made from egg shell powder for Congo red [J]. Inorganic Chemicals Industry, 2021, 53(8): 91-95. |
| [13] | Zhao Li,Zhuo Minquan,Gong Fuzhong,Wang Jun,Ruan Heng,Li Kaicheng,Li Yanlin. Synthesis of vaterite CaCO3 microspheres by carbonization method and its formation mechanism [J]. Inorganic Chemicals Industry, 2021, 53(3): 38-43. |
| [14] | XIE Juan,LIU Songlin,TAO Shaocheng,HU Houmei,LIU Xu. Study on preparation of magnesium carbonate hydroxide by carbonization method from high-Mg phosphate tailings [J]. Inorganic Chemicals Industry, 2021, 53(12): 135-139. |
| [15] | XU Daying,GENG Wenjuan,QIAN Dequan,XUE Rui. Influence of several carbohydrates on crystallization of nano calcium carbonate [J]. Inorganic Chemicals Industry, 2021, 53(11): 77-80. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||