Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (11): 71-78.doi: 10.19964/j.issn.1006-4990.2022-0348
• Research & Development • Previous Articles Next Articles
Study on natural evaporation experimental of deep brine in Yahu structure
WU Liping1,2,YUAN Hongzhan1,2,JIN Fang1,2
- 1. Qinghai Provincial Key Laboratory of Salt Lake Resources Exploration and Research in Qaidam Basin,Golmud 816099,China
2. Qinghai Qaidam comprehensive geological and Mineral Exploration Institute
-
Received:2022-07-12Online:2022-11-10Published:2022-11-23 -
Contact:YUAN Hongzhan
CLC Number:
Cite this article
WU Liping,YUAN Hongzhan,JIN Fang. Study on natural evaporation experimental of deep brine in Yahu structure[J]. Inorganic Chemicals Industry, 2022, 54(11): 71-78.
share this article
Table 2
Testing methods and instruments of sample"
| 元素 | 检测方法 | 使用仪器 |
|---|---|---|
| K+、Na+、Ca2+、Mg2+、SO42-、Li+、Sr2+ | 电感耦合等离子体发射光谱法 | Agilent 5800型ICP-OES |
| Cl- | 硝酸银容量法 | 50 mL酸式滴定管 |
| B2O3 | 容量法 | 50 mL酸式滴定管 |
| Rb+、Cs+ | 电感耦合等离子体质谱法 | Agilent 7800型ICP-MS |
| Br-、I- | 分光光度法 | P9PC型紫外可见分光光度计 |
| pH | pH玻璃电极法 | PHSJ-4A型pH计 |
| 密度 | 比重瓶法 | MS105型电子天平 |
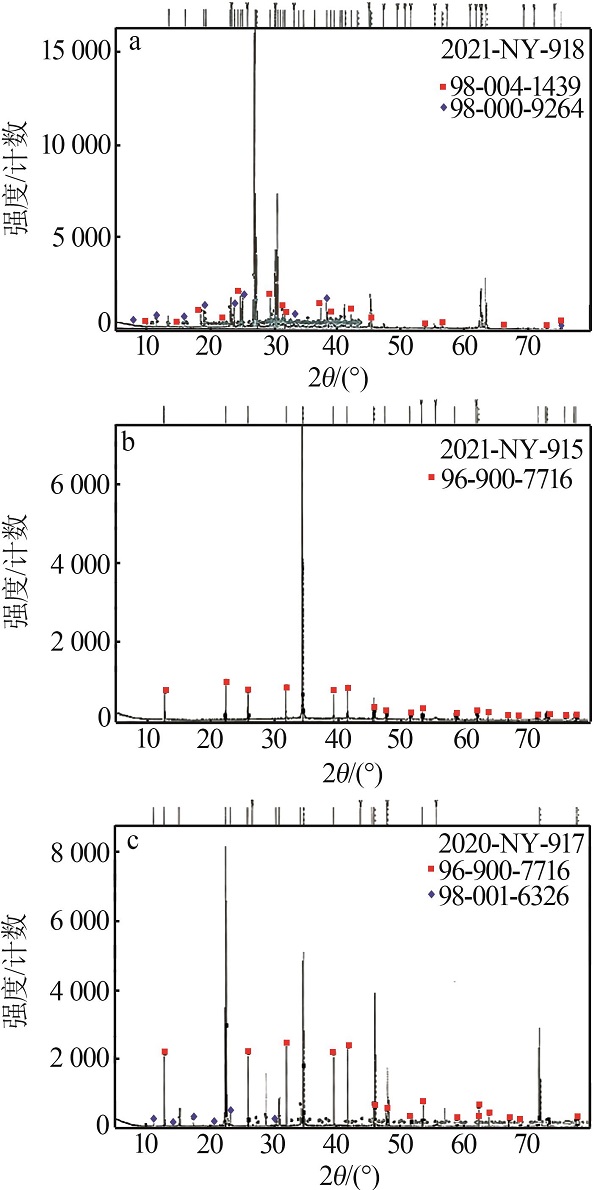
| 矿物类型 | X射线衍射(XRD)分析 | D8 Discover型X衍射分析仪 |
Table 3
Daily observation results"
| 取样日期 | 卤温/ ℃ | 密度/ (g.mL-1) | pH | 矿化度/(g.L-1) | 液样编号 | 固样编号 |
|---|---|---|---|---|---|---|
| 2020.07.05 | 22.00 | 1.080 9 | 7.13 | 121.6 | YL0 | — |
| 2020.09.02 | 16.50 | 1.189 7 | 8.60 | 287.1 | YL1 | YS1 |
| 2020.09.06 | 16.00 | 1.208 3 | 8.14 | 329.9 | YL2 | YS2 |
| 2020.09.08 | 20.00 | 1.208 5 | 8.16 | 325.5 | YL3 | YS3 |
| 2020.10.13 | 15.90 | 1.217 3 | 8.45 | 326.8 | YL5 | YS5 |
| 2021.01.28 | 4.20 | 1.236 8 | 8.01 | 334.6 | YL9 | YS9、YS9′ |
| 2021.03.03 | 7.80 | 1.253 3 | 8.35 | 361.5 | YL10 | YS10 |
| 2021.03.21 | 10.10 | 1.268 2 | 8.28 | 376.2 | YL11 | YS11 |
| 2021.04.05 | 21.50 | 1.274 6 | 8.20 | 397.9 | YL12 | YS12 |
| 2021.04.15 | 17.00 | 1.294 1 | 8.17 | 418.7 | YL13 | YS13、YS13′ |
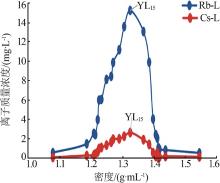
| 2021.04.27 | 19.00 | 1.329 3 | 8.07 | 473.7 | YL15 | YS15 |
| 2021.05.03 | 9.00 | 1.360 0 | — | 495.0 | YL16 | YS16、YS16′ |
| 2021.05.06 | 13.90 | 1.372 0 | 8.01 | 536.8 | YL17 | YS17 |
| 2021.05.08 | 19.60 | 1.390 3 | 8.00 | 572.1 | YL18 | YS18 |
| 2021.05.12 | 17.60 | 1.411 6 | 7.95 | 600.3 | YL20 | YS20、YS20′ |
| 2021.05.21 | 14.00 | 1.416 4 | 7.97 | 607.4 | YL21 | YS21 |
| 2021.05.26 | 15.30 | 1.423 6 | 7.71 | 606.7 | YL23 | YS23、YS23′ |
| 2021.06.03 | 18.30 | 1.442 7 | 7.68 | 646.8 | YL24 | YS24 |
| 2021.07.02 | 19.80 | 1.464 4 | 7.65 | 696.0 | YL25 | YS25 |
| 2021.07.12 | 21.30 | 1.454 4 | 7.58 | 677.0 | YL26 | YS26 |
| 2021.07.19 | 28.50 | 1.498 6 | 7.58 | 759.6 | YL27 | YS27 |
| 2021.07.31 | 23.00 | 1.512 5 | — | 774.3 | YL28 | YS28 |
| 2021.08.03 | 22.00 | 1.551 6 | 7.95 | 781.4 | YL29 | YS29 |
Table 4
Test results of main elements in liquid samples"
液样 编号 | 各离子的质量浓度/(g·L-1) | 相图指数/(10-2 g·g-1) | 相图指数/(10-2 g·g-1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | Cl- | SO42- | KCl | NaCl | MgCl2 | KCl | MgCl2 | CaCl2 | |||
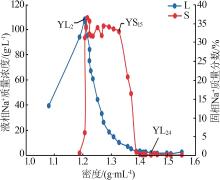
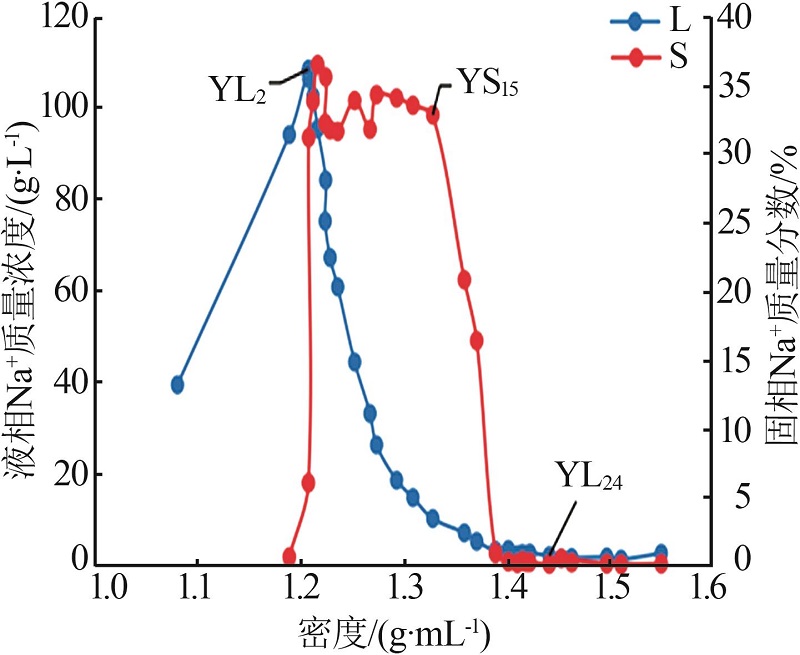
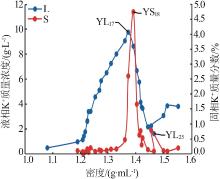
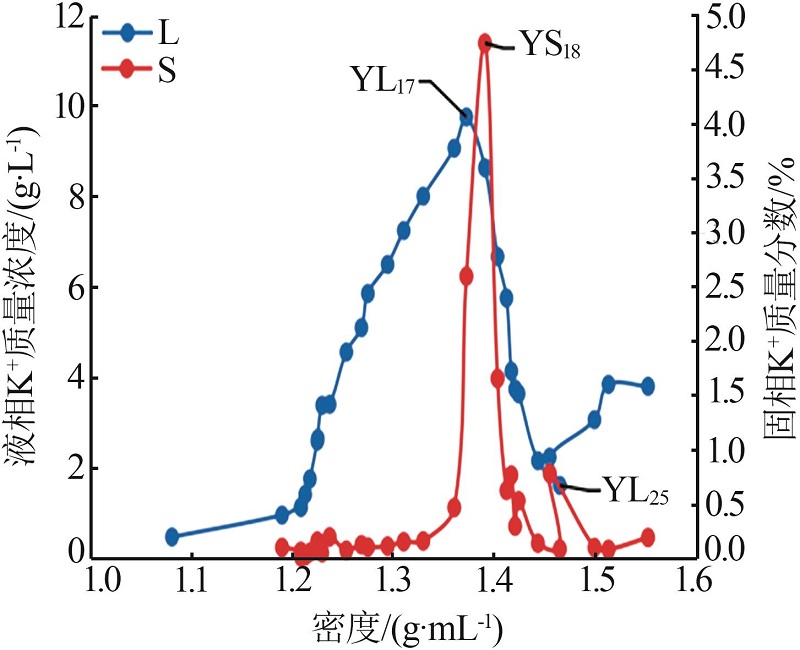
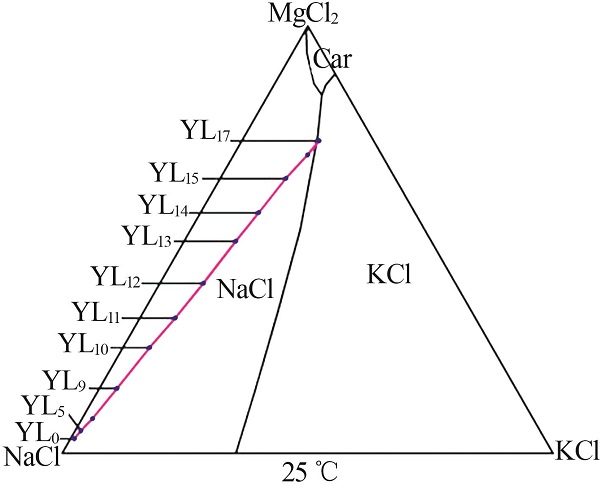
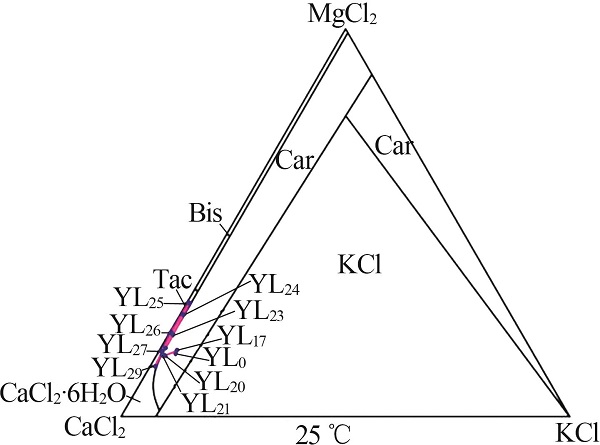
| YL0 | 0.476 | 39.36 | 6.076 | 0.869 | 73.32 | 0.699 | 0.87 | 95.87 | 3.26 | 4.30 | 16.11 | 79.60 | ||
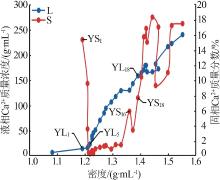
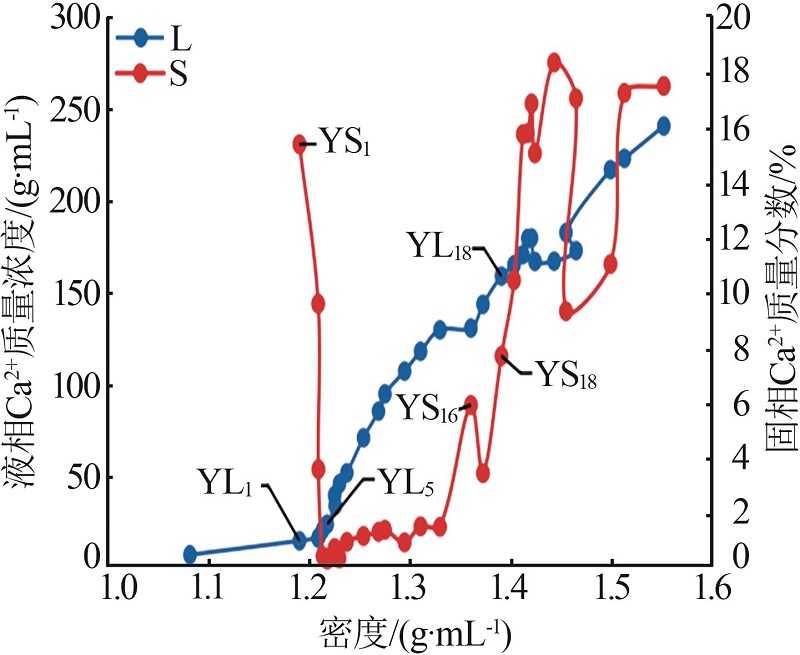
| YL1 | 0.960 | 94.03 | 13.67 | 1.994 | 173.5 | 1.120 | 0.74 | 96.12 | 3.14 | 3.85 | 16.44 | 79.71 | ||
| YL2 | 1.141 | 108.4 | 15.14 | 2.372 | 199.9 | 0.965 | 0.76 | 96.00 | 3.24 | 4.08 | 17.41 | 78.52 | ||
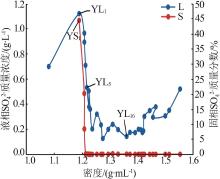
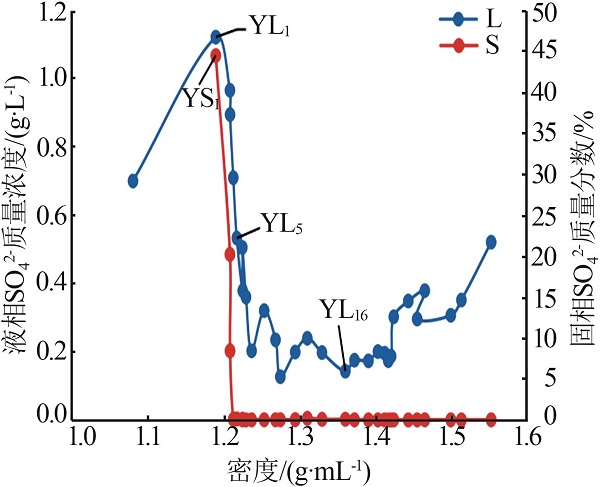
| YL5 | 1.760 | 95.36 | 22.74 | 3.449 | 200.0 | 0.532 | 1.29 | 93.49 | 5.21 | 4.20 | 16.92 | 78.87 | ||
| YL9 | 3.410 | 60.76 | 50.65 | 7.409 | 205.5 | 0.202 | 3.42 | 81.30 | 15.28 | 3.70 | 16.51 | 79.79 | ||
| YL13 | 6.506 | 18.50 | 106.0 | 14.90 | 259.9 | 0.199 | 10.53 | 39.92 | 49.55 | 3.41 | 16.02 | 80.57 | ||
| YL15 | 8.016 | 10.12 | 128.4 | 18.95 | 293.2 | 0.197 | 13.26 | 22.33 | 64.41 | 3.43 | 16.68 | 79.89 | ||
| YL16 | 9.079 | 6.990 | 129.4 | 20.52 | 311.6 | 0.142 | 14.99 | 15.39 | 69.62 | 3.80 | 17.63 | 78.58 | ||
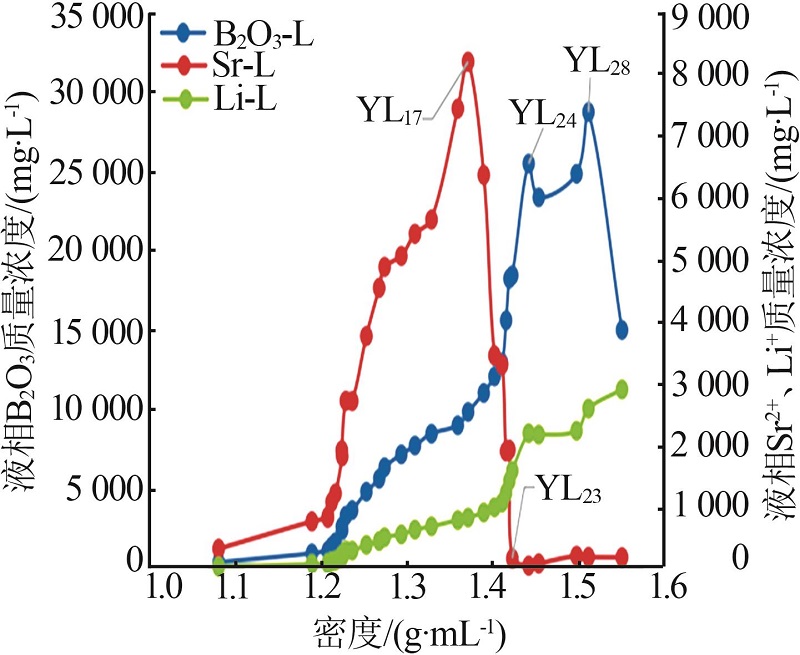
| YL17 | 9.769 | 5.130 | 142.3 | 21.92 | 338.6 | 0.175 | 15.85 | 11.09 | 73.06 | 3.73 | 17.22 | 79.04 | ||
| YL18 | 8.645 | 3.147 | 157.7 | 22.80 | 361.3 | 0.173 | 14.48 | 7.03 | 78.49 | 3.04 | 16.47 | 80.50 | ||
| YL20 | 5.765 | 2.209 | 169.4 | 22.77 | 382.7 | 0.195 | 10.39 | 5.31 | 84.30 | 1.93 | 15.67 | 82.40 | ||
| YL21 | 4.144 | 2.481 | 178.2 | 24.55 | 379.1 | 0.173 | 7.16 | 5.72 | 87.13 | 1.32 | 16.09 | 82.58 | ||
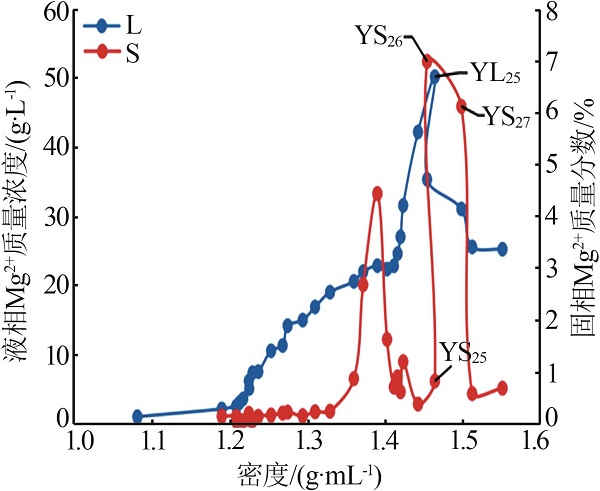
| YL23 | 3.646 | 2.577 | 165.7 | 31.55 | 382.7 | 0.302 | 5.07 | 4.78 | 90.15 | 1.18 | 20.97 | 77.85 | ||
| YL24 | 2.163 | 1.997 | 165.8 | 42.21 | 406.5 | 0.348 | 2.36 | 2.91 | 94.73 | 0.66 | 26.30 | 73.04 | ||
| YL25 | 1.617 | 1.585 | 171.5 | 50.24 | 442.1 | 0.378 | 1.51 | 1.98 | 96.51 | 0.46 | 29.16 | 70.38 | ||
| YL26 | 2.243 | 1.488 | 181.3 | 35.33 | 430.7 | 0.295 | 2.92 | 2.58 | 94.50 | 0.66 | 21.46 | 77.87 | ||
| YL27 | 3.071 | 1.710 | 215.6 | 31.04 | 480.6 | 0.306 | 4.44 | 3.30 | 92.26 | 0.81 | 16.78 | 82.41 | ||
| YL28 | 3.852 | 1.257 | 221.8 | 25.52 | 490.1 | 0.351 | 6.65 | 2.89 | 90.46 | 1.02 | 13.86 | 85.12 | ||
| YL29 | 3.809 | 2.515 | 239.3 | 25.19 | 491.9 | 0.520 | 6.46 | 5.69 | 87.84 | 0.94 | 12.84 | 86.22 | ||
Table 5
Test results of trace elements in liquid samples"
| 液样编号 | 各物质质量浓度/(mg·L-1) | ||||||
|---|---|---|---|---|---|---|---|
| B2O3 | Li+ | Sr2+ | Rb+ | Cs+ | Br- | I- | |
| YL0 | 403.7 | 29.50 | 321.2 | 0.609 | 0.115 | 57.37 | 22.15 |
| YL1 | 955.3 | 70.75 | 762.8 | 1.517 | 0.311 | — | — |
| YL2 | 1 117 | 78.34 | 820.4 | — | — | — | — |
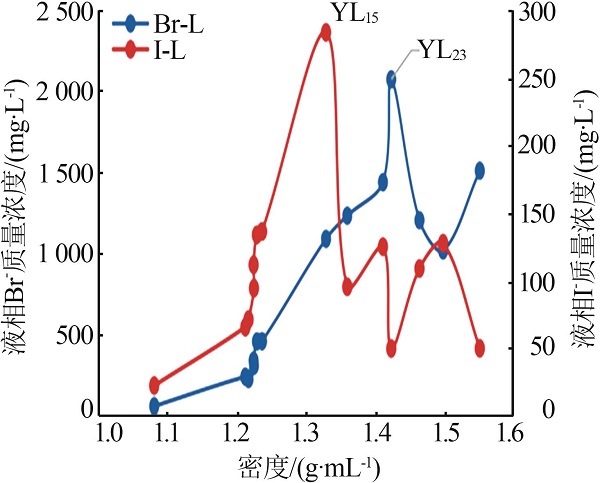
| YL5 | 1 660 | 128.1 | 1 215 | 2.450 | 0.483 | 224.90 | 71.08 |
| YL9 | 3 680 | 290.8 | 2 711 | 6.077 | 1.134 | 458.60 | 136.4 |
| YL13 | 7 187 | 553.0 | 5 060 | 11.17 | 2.027 | — | — |
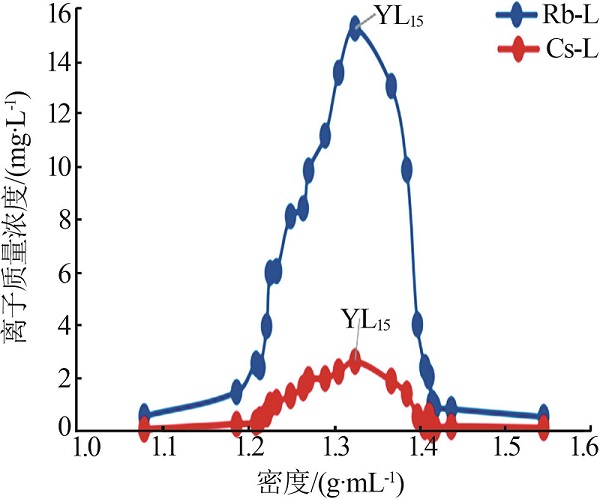
| YL15 | 8 488 | 683.3 | 5 649 | 15.21 | 2.669 | 1 091 | 284.1 |
| YL16 | 9 018 | 784.3 | 7 446 | — | — | 1 234 | 95.39 |
| YL17 | 9 858 | 825.3 | 8 205 | 13.05 | 1.937 | — | — |
| YL18 | 11 052 | 907.4 | 6 372 | 9.891 | 1.451 | — | — |
| YL20 | 12 952 | 1 069 | 3 298 | 2.459 | 0.214 | 1 441 | 125.1 |
| YL21 | 15 626 | 1 231 | 1 898 | 2.097 | 0.632 | — | — |
| YL23 | 18 461 | 1 584 | 163.6 | 0.950 | 0.158 | 2 077 | 49.58 |
| YL24 | 25 498 | 2 191 | 47.12 | 0.876 | 0.217 | — | — |
| YL25 | 25 908 | 2 641 | 23.34 | — | — | 1 206 | 108.9 |
| YL28 | 28 740 | 2 590 | 187.7 | — | — | — | — |
| YL29 | 15 015 | 2 899 | 181.8 | — | — | 1 512 | 49.72 |
Table 6
Test results of solid samples"
固样 编号 | 各物质的质量分数/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | Cl- | SO42- | B2O3 | Li+ | Sr2+ | |
| YS1 | 0.10 | 0.58 | 15.29 | 0.13 | 0.00 | 44.43 | 0.008 | 0.000 | 0.14 |
| YS3 | 0.014 | 31.14 | 3.51 | 0.020 | 48.20 | 8.42 | 0.007 | 0.001 | 0.041 |
| YS5 | 0.057 | 36.45 | 0.23 | 0.029 | 56.99 | 0.12 | 0.018 | 0.001 | 0.010 |
| YS9 | 0.20 | 31.58 | 0.87 | 0.12 | 50.59 | 0.012 | 0.060 | 0.008 | 0.052 |
| YS9′ | 0.10 | 36.72 | 0.36 | 0.035 | 57.67 | 0.17 | 0.021 | 0.005 | 0.020 |
| YS10 | 0.076 | 33.83 | 1.09 | 0.15 | 55.31 | 0.021 | 0.089 | 0.006 | 0.060 |
| YS11 | 0.13 | 31.74 | 1.23 | 0.18 | 52.17 | 0.018 | 0.092 | 0.007 | 0.075 |
| YS12 | 0.11 | 34.26 | 1.31 | 0.20 | 55.54 | 0.024 | 0.086 | 0.008 | 0.075 |
| YS13 | 0.11 | 34.02 | 0.85 | 0.13 | 55.20 | 0.033 | 0.070 | 0.005 | 0.046 |
| YS13′ | 0.12 | 34.14 | 1.12 | 0.17 | 55.08 | 0.13 | 0.074 | 0.006 | 0.059 |
| YS15 | 0.16 | 32.78 | 1.42 | 0.22 | 54.24 | 0.069 | 0.10 | 0.008 | 0.085 |
| YS16 | 0.47 | 20.77 | 5.84 | 0.85 | 45.02 | 0.075 | 0.32 | 0.029 | 0.33 |
| YS16′ | 0.057 | 35.69 | 0.76 | 0.10 | 57.04 | 0.15 | 0.051 | 0.004 | 0.050 |
| YS17 | 2.60 | 16.34 | 3.37 | 2.67 | 43.05 | 0.014 | 0.19 | 0.020 | 0.18 |
| YS18 | 4.75 | 0.83 | 7.62 | 4.44 | 31.53 | 0.010 | 0.24 | 0.024 | 2.41 |
| YS20 | 0.63 | 0.10 | 15.66 | 0.69 | 31.12 | 0.006 | 0.16 | 0.016 | 1.98 |
| YS20ˊ′ | 1.81 | 0.85 | 11.20 | 2.26 | 30.53 | 0.026 | 0.46 | 0.042 | 2.04 |
| YS21 | 0.77 | 0.27 | 15.70 | 0.89 | 31.80 | 0.001 | 0.20 | 0.017 | 1.52 |
| YS23 | 0.53 | 0.17 | 14.97 | 1.18 | 30.54 | 0.008 | 0.58 | 0.046 | 0.076 |
| YS23′ | 1.11 | 0.57 | 15.32 | 1.18 | 32.45 | 0.026 | 0.27 | 0.020 | 0.95 |
| YS24 | 0.14 | 0.049 | 18.26 | 0.36 | 33.36 | 0.003 | 0.28 | 0.016 | 0.025 |
| YS25 | 0.088 | 0.15 | 16.96 | 0.80 | 31.86 | 0.019 | 0.59 | 0.042 | 0.027 |
| YS26 | 0.78 | 0.46 | 9.24 | 7.00 | 37.94 | 0.009 | 0.48 | 0.035 | 0.035 |
| YS27 | 0.10 | 0.063 | 10.95 | 6.12 | 37.16 | 0.013 | 0.81 | 0.068 | 0.068 |
| YS28 | 0.085 | 0.022 | 17.15 | 0.56 | 32.05 | 0.007 | 0.54 | 0.043 | 0.043 |
| YS29 | 0.19 | 0.068 | 17.41 | 0.67 | 32.51 | 0.012 | 3.87 | 0.057 | 0.027 |
| 1 | 邓天龙,王士强,郭亚飞.柴达木盆地盐湖卤水体系介稳相平衡与相图[M].北京:科学出版社,2017. |
| DENG Tianlong, WANG Shiqiang, GUO Yafei.Metastable phase equilibrium and phase diagram of salt lake brine system in Qaidam Basin[M].Beijing:Science Press,2017. | |
| 2 | 郑绵平,邓天龙,阿哈龙·奥伦.盐湖科学概论[M].北京:科学出版社,2018. |
| ZHENG Mianping, DENG Tianlong, Oren A.Introduction to salt lake science[M].Beijing:Science Press,2018. | |
| 3 | 穆延宗,乜贞,卜令忠,等.我国油(气)田水钾资源研究进展[J].地球科学进展,2016,31(2):147-160. |
| MU Yanzong, NIE Zhen, BU Lingzhong, et al.Progress in study of potash resources of oil(gas) field brine in China[J].Advances in Earth Science,2016,31(2):147-160. | |
| 4 | 中华人民共和国自然资源部. DZ/T 0212.1—2020 矿产地质勘查规范盐类 [S].北京:中国标准出版社,2020. |
| Ministry of natural resources of the People's Republic of China. DZ/T 0212.1—2020 Code for mineral geological exploration salt [S].Beijing:China Standards Press,2020. | |
| 5 | 刘颖,王云生,乜贞,等.柴西深层地下卤水资源及其综合利用研究进展[J].无机盐工业,2018,50(1):12-15,27. |
| LIU Ying, WANG Yunsheng, NIE Zhen, et al.Research progress on comprehensive utilization of deep underground brine resources in western Qaidam Basin[J].Inorganic Chemicals Industry,2018,50(1):12-15,27. | |
| 6 | 赵为永,魏学斌,雷涛,等.青海省南翼山构造深层卤水矿床特征及分布规律研究[J].能源与环保,2021,43(5):39-45. |
| ZHAO Weiyong, WEI Xuebin, LEI Tao, et al.Study on deposit characteristics and distribution of deep brine in Nanyishan,Qinghai Province[J].China Energy and Environmental Protection,2021,43(5):39-45. | |
| 7 | 李洪普,侯献华,郑绵平,等.柴达木盆地西部更新统砂砾型深层卤水钾矿成矿模式与找矿方向探讨[J].湖泊科学,2022,34(3):1043-1054. |
| LI Hongpu, HOU Xianhua, ZHENG Mianping, et al.Discussion on metallogenic model and prospecting direction of Pleistocene gravel brine Potassium deposit in western Qaidam Basin[J].Journal of Lake Sciences,2022,34(3):1043-1054. | |
| 8 | 刘溪溪,岳鑫,袁文虎,等.柴达木盆地西部狮子沟背斜构造区深部卤水水化学特征及演化分析[J].盐湖研究,2019,27(1):73-81. |
| LIU Xixi, YUE Xin, YUAN Wenhu, et al.Hydrochemical characteristics and evolutionary process of deep brines from shizigou anticline structure in Qaidam Basin,China[J].Journal of Salt Lake Research,2019,27(1):73-81. | |
| 9 | 韩光,樊启顺,刘久波,等.柴达木盆地中西部背斜构造深层卤水水化学特征与成因[J].盐湖研究,2021,29(4):1-11. |
| HAN Guang, FAN Qishun, LIU Jiubo, et al.Origin and hydrochemistry of deep brines from anticlinal reservoir in the western-central Qaidam Basin[J].Journal of Salt Lake Research,2021,29(4):1-11. | |
| 10 | 侯献华,冯磊,郑绵平,等.南翼山富钾锂卤水储层识别方法[J].地球科学,2022,47(1):45-55. |
| HOU Xianhua, FENG Lei, ZHENG Mianping, et al.Recognition method of potassium-rich lithium brine reservoir in Nanyishan[J].Earth Science,2022,47(1):45-55. | |
| 11 | 徐凯,许建新,常政,等.柴达木盆地南翼山油田卤水水化学及氢氧同位素地球化学特征[J].盐湖研究,2021,29(4):43-51. |
| XU Kai, XU Jianxin, CHANG Zheng, et al.Hydrochemical,hydrogen and oxygen isotopic characteristics of nanyishan oilfield brine,Qaidam Basin[J].Journal of Salt Lake Research,2021,29(4):43-51. | |
| 12 | 郭璞,潘洪峰,侯安平,等.南翼山构造主要地质特征及油气成藏模式[J].青海石油,2010,28(2):7-14. |
| GUO Pu, PAN Hongfeng, HOU Anping, et al.Main geological characteristics and hydrocarbon accumulation model of Nanyishan structure[J].Qinghai Petroleum,2010,28(2):7-14. | |
| 13 | 靳芳,李洪普,常东海.柴达木盆地南翼山背斜构造区卤水自然蒸发实验[J].无机盐工业,2021,53(11):86-90. |
| JIN Fang, LI Hongpu, CHANG Donghai.Experiment on natural evaporation of brine in Nanyishan anticline structural area of Qaidam Basin[J].Inorganic Chemicals Industry,2021,53(11):86-90. | |
| 14 | 靳芳,李洪普,武丽平,等.柴达木盆地深层卤水自然蒸发试验[J].化工矿物与加工,2022,51(5):54-58. |
| JIN Fang, LI Hongpu, WU Liping, et al.The natural evaporation experiments with deep brines from Qaidam Basin[J].Industrial Minerals & Processing,2022,51(5):54-58. | |
| 15 | 唐发满,杨红军,张生鹏,等.一里坪盐湖夏季卤水25 ℃等温蒸发[J].无机盐工业,2021,53(10):52-58. |
| TANG Faman, YANG Hongjun, ZHANG Shengpeng, et al.Isothermal evaporation of summer brine of Yiliping salt lake at 25 ℃[J].Inorganic Chemicals Industry,2021,53(10):52-58. | |
| 16 | 乔东慧.柴达木盆地东台吉乃尔盐湖卤水蒸发实验研究[D].成都:成都理工大学,2012. |
| QIAO Donghui.Evaporation studies for the salt lake brine in the dongtaijinaier salt lake,Qaidam Basin[D].Chengdu:Chengdu University of Technology,2012. | |
| 17 | 彭玲玲,魏学斌,赵为永,等.黑北凹地富钾地下卤水自然蒸发实验研究[J].无机盐工业,2019,51(6):11-16. |
| PENG Lingling, WEI Xuebin, ZHAO Weiyong, et al.Study on natural evaporation of underground potassium-rich brine in Heibei concave[J].Inorganic Chemicals Industry,2019,51(6):11-16. | |
| 18 | T/QAS 025—2021 卤水-pH的测定-玻璃电极法[S].青海:青海省标准化协会,2021. |
| 19 | 邓天龙,周桓,陈侠.水盐体系相图及应用[M].北京:化学工业出版社,2020. |
| DENG Tianlong, ZHOU Huan, CHEN Xia.Salt-water system phase diagrams and applications[M].BeiJing:Chemical Industry Press,2020. | |
| 20 | 本山正夫,门田稔,冈俊平.NaCl-KCl-MgCl2-CaCl2-H2O体系的25 ℃相平衡[J].日本海水学会会刊,1972,26(4):173-180 . |
| 21 | 程怀德,李俊,海擎宇.白垩纪海水Ca异常与溢晶石形成的关系[J].化工矿产地质,2018,40(3):129-140. |
| CHENG Huaide, LI Jun, Qingyu HAI.Relationship between Cretaceous seawater Ca anomaly and tachyhydrite formation[J].Geology of Chemical Minerals,2018,40(3):129-140. | |
| 22 | 程怀德,马海州,山发寿,等.基于相化学研究老挝万象钾镁盐矿床形成的机制[J].地球学报,2010,31(2):194-202. |
| CHENG Huaide, MA Haizhou, SHAN Fashou, et al.A study of the formation mechanism of the Vientiane potash deposit based on phase chemistry[J].Acta Geoscientica Sinica,2010,31(2):194-202. |
| [1] | YU Xudong, LI Jing, REN Siying, LUO Jun, ZENG Ying. Study on solid-liquid phase equilibrium of Li+,K+,Ca2+//Cl--H2O quaternary system at 298.2 K [J]. Inorganic Chemicals Industry, 2025, 57(3): 30-35. |
| [2] | JIN Fang,LI Hongpu,CHANG Donghai. Experiment on natural evaporation of brine in Nanyishan anticline structural area of Qaidam Basin [J]. Inorganic Chemicals Industry, 2021, 53(11): 86-90. |
| [3] | BIAN Shaoju, XU Naicai, ZHANG Yongxing, LI Wu. Crystallizing sequence of salts and enrichment rule of boron and lithium during natural evaporation of Manasi salt lake brine [J]. Inorganic Chemicals Industry, 2021, 53(11): 55-59. |
| [4] | Peng Lingling,Wei Xuebin,Zhao Weiyong,Liu Ying,Mu Yanzong,Nie Zhen,Wang Yunsheng. Study on natural evaporation of underground potassium-rich brine in Heibei concave [J]. Inorganic Chemicals Industry, 2019, 51(6): 11-16. |
| [5] | TAN Xiu-Min, ZHANG Xiu-Feng, ZHANG Li-Zhen. Study on bromine extraction technology of Jiangling depression deep brine [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(5): 13-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||