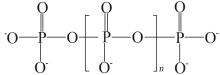
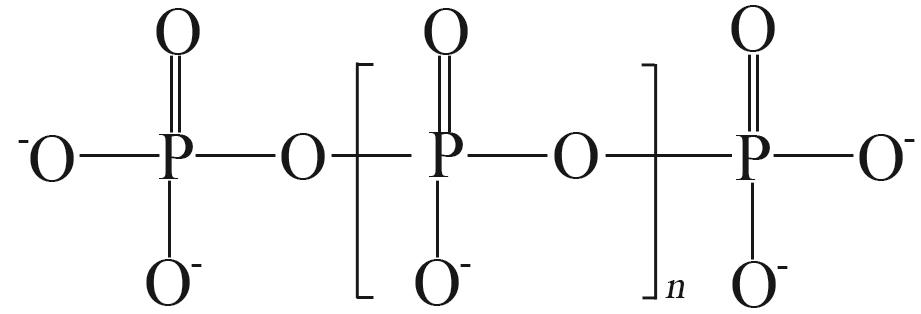
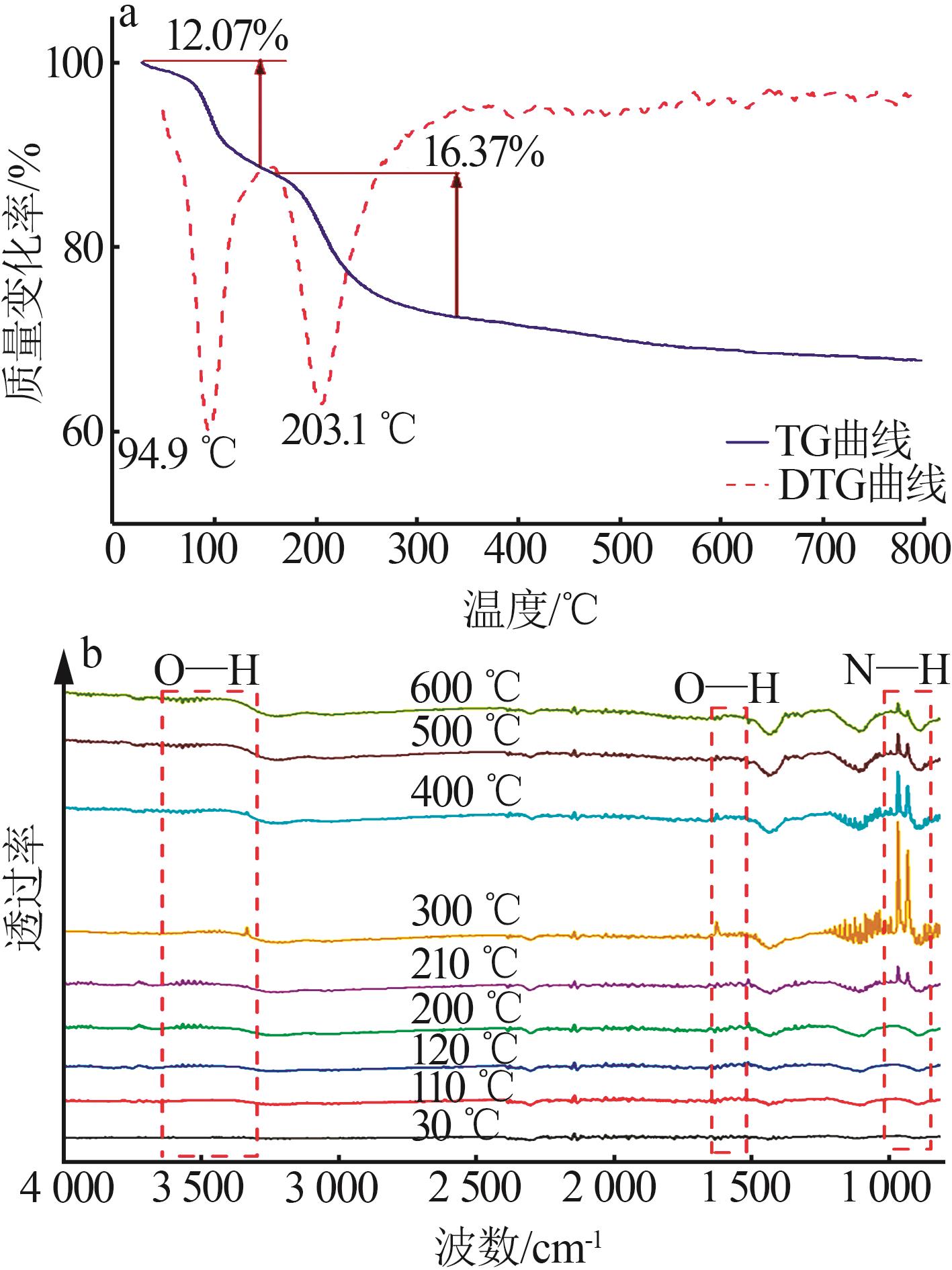
| [1] |
况新亮,刘垂祥,熊朋.锂离子电池产业分析及市场展望[J].无机盐工业,2022,54(8):12-19,32.
|
|
KUANG Xinliang, LIU Chuixiang, XIONG Peng.Industry analysis and market prospect of lithium ion battery[J].Inorganic Chemicals Industry,2022,54(8):12-19,32.
|
| [2] |
王紫涵,李军,陈明,等.硝酸铁和磷酸共沉淀法制备电池级磷酸铁工艺研究[J].无机盐工业,2023,55(7):51-57.
|
|
WANG Zihan, LI Jun, CHEN Ming,et al.Study on preparation of battery grade ferric phosphate by co-precipitation of ferric nitrate and phosphoric acid[J].Inorganic Chemicals Industry,2023,55(7):51-57.
|
| [3] |
王君婷,马航,查坐统,等.磷酸铁工业废水处理工艺研究进展[J].无机盐工业,2024,56(6):26-33.
|
|
WANG Junting, MA Hang, ZHA Zuotong,et al.Research progress of iron phosphate industrial wastewater treatment process[J].Inorganic Chemicals Industry,2024,56(6):26-33.
|
| [4] |
王维,李伟,李立敏,等.磷酸铁生产废水回用及零排放的预处理研究[J].无机盐工业,2024,56(8):99-103.
|
|
WANG Wei, LI Wei, LI Limin,et al.Study on pretreatment of iron phosphate production wastewater reuse and zero discharge[J].Inorganic Chemicals Industry,2024,56(8):99-103.
|
| [5] |
吴航,张丽芳,白威,等.聚合温度对聚磷酸钙聚合度及结构的影响[J].无机材料学报,2012,27(2):174-178.
|
|
WU Hang, ZHANG Lifang, BAI Wei,et al.Effect of polymerization temperature on polymerization degree and structure of calcium polyphosphate[J].Journal of Inorganic Materials,2012,27(2):174-178.
|
| [6] |
赵蕾蕾,舒艺周,漆增连,等.不同APP添加量对复合肥料造粒性能的影响[J].无机盐工业,2024,56(8):74-82.
|
|
ZHAO Leilei, SHU Yizhou, QI Zenglian,et al.Effect of different APP addition on granulation performance of compound fertilizer[J].Inorganic Chemicals Industry,2024,56(8):74-82.
|
| [7] |
袁太艳,严正娟,黄成东,等.聚磷酸铵在紫色土壤中的吸附-解吸特征[J].浙江农业学报,2023,35(2):403-416.
|
|
YUAN Taiyan, YAN Zhengjuan, HUANG Chengdong,et al.Adsorption-desorption characteristics of ammonium polyphosphate in purple soils[J].Acta Agriculturae Zhejiangensis,2023,35(2):403-416.
|
| [8] |
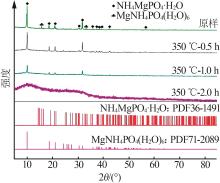
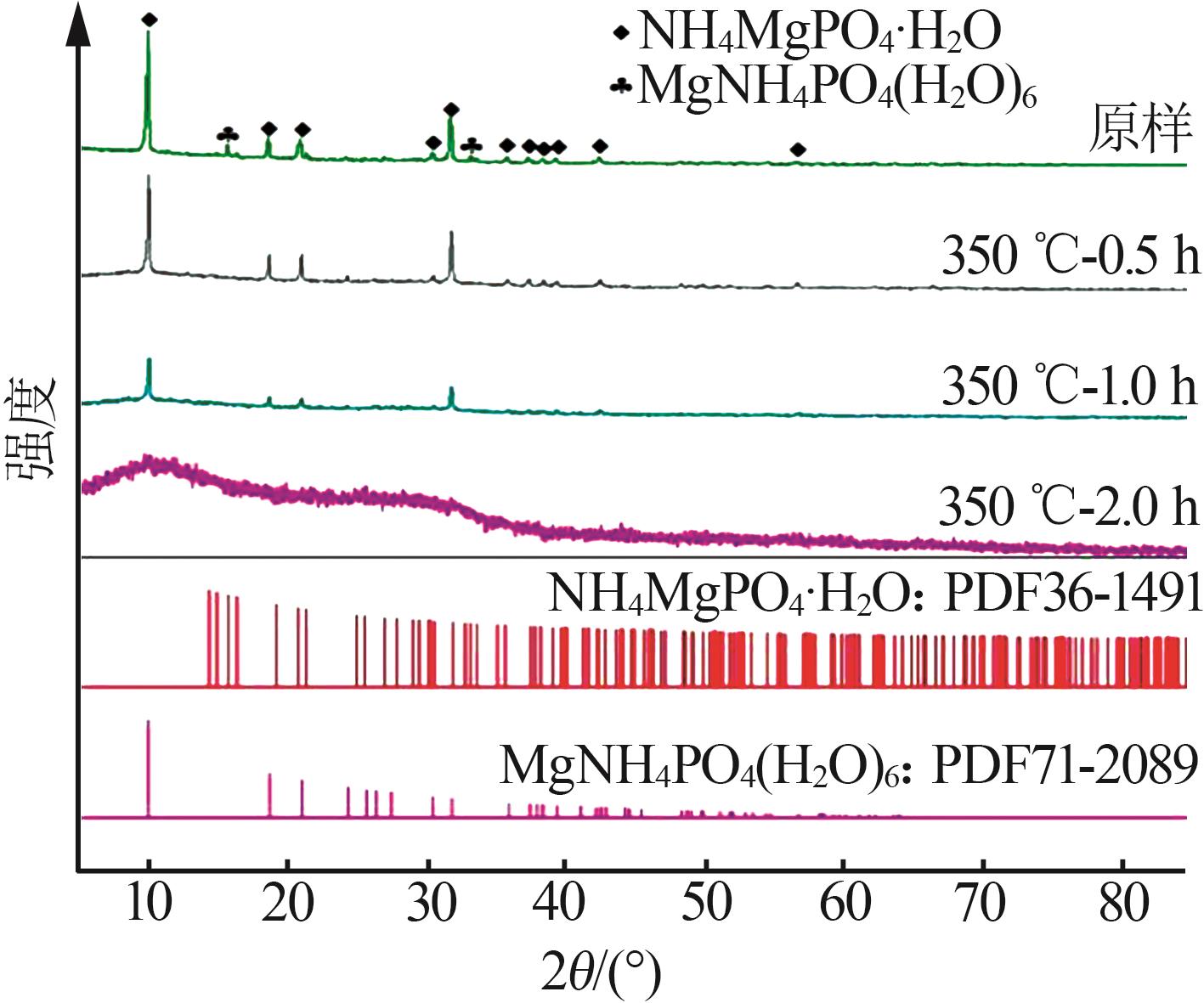
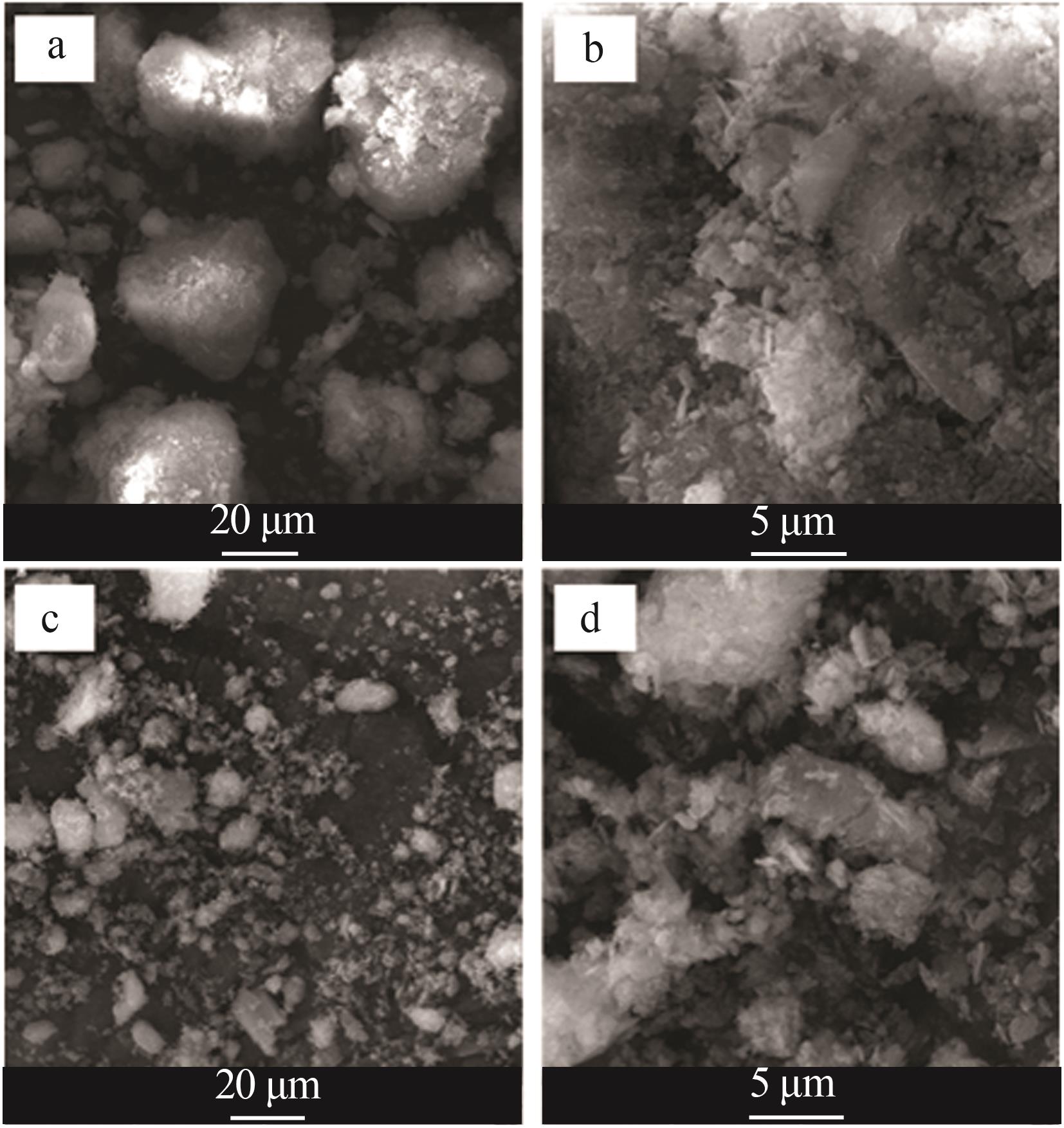
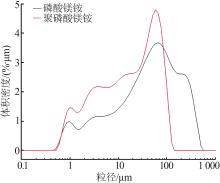
苗林平,许淼,王辛龙,等.聚磷酸钙镁的制备研究[J].无机盐工业,2022,54(9):63-68.
|
|
MIAO Linping, XU Miao, WANG Xinlong,et al.Study on preparation of calcium magnesium polyphosphate[J].Inorganic Chemicals Industry,2022,54(9):63-68.
|
| [9] |
刘续,汤建伟,刘咏,等.水溶性农用聚磷酸铵的研究与应用进展[J].无机盐工业,2020,52(12):7-11.
|
|
LIU Xu, TANG Jianwei, LIU Yong,et al.Research and application progress of water-soluble agricultural ammonium polyphosphate[J].Inorganic Chemicals Industry,2020,52(12):7-11.
|
| [10] |
YANG Jingxu, XIE Wenji, KONG Xingjian,et al.Reactive extrusion of ammonium polyphosphate in a twin-screw extruder:Polydispersity improvement[J].Chemical Engineering and Processing-Process Intensification,2018,133:58-65.
|
| [11] |
XIE Wenji, WANG Xinlong, LI Yongsheng,et al.Simultaneous determination of various phosphates in water-soluble ammonium polyphosphate[J].Chromatographia,2019,82(11):1687-1695.
|
| [12] |
王娇,杜昌文,申亚珍,等.中红外光声光谱法测定土壤顶空氨气浓度[J].土壤,2014,46(6):1017-1023.
|
|
WANG Jiao, DU Changwen, SHEN Yazhen,et al.Measurement of ammonia in soil headspace by mid-infrared photoacoustic spectroscopy[J].Soils,2014,46(6):1017-1023.
|
| [13] |
曾庆玲,王露,沈春花,等.鸟粪石循环利用处理高氨氮废水的热解行为[J].环境工程学报,2013,7(7):2541-2546.
|
|
ZENG Qingling, WANG Lu, SHEN Chunhua,et al.Thermal decomposition behavior of struvite in recycling treatment of high ammonia-nitrogen wastewater[J].Chinese Journal of Environmental Engineering,2013,7(7):2541-2546.
|
 ), XU Dehua(
), XU Dehua( ), SHOU Zhixin, YANG Wengong, WANG Xinlong
), SHOU Zhixin, YANG Wengong, WANG Xinlong