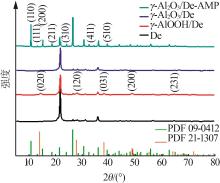
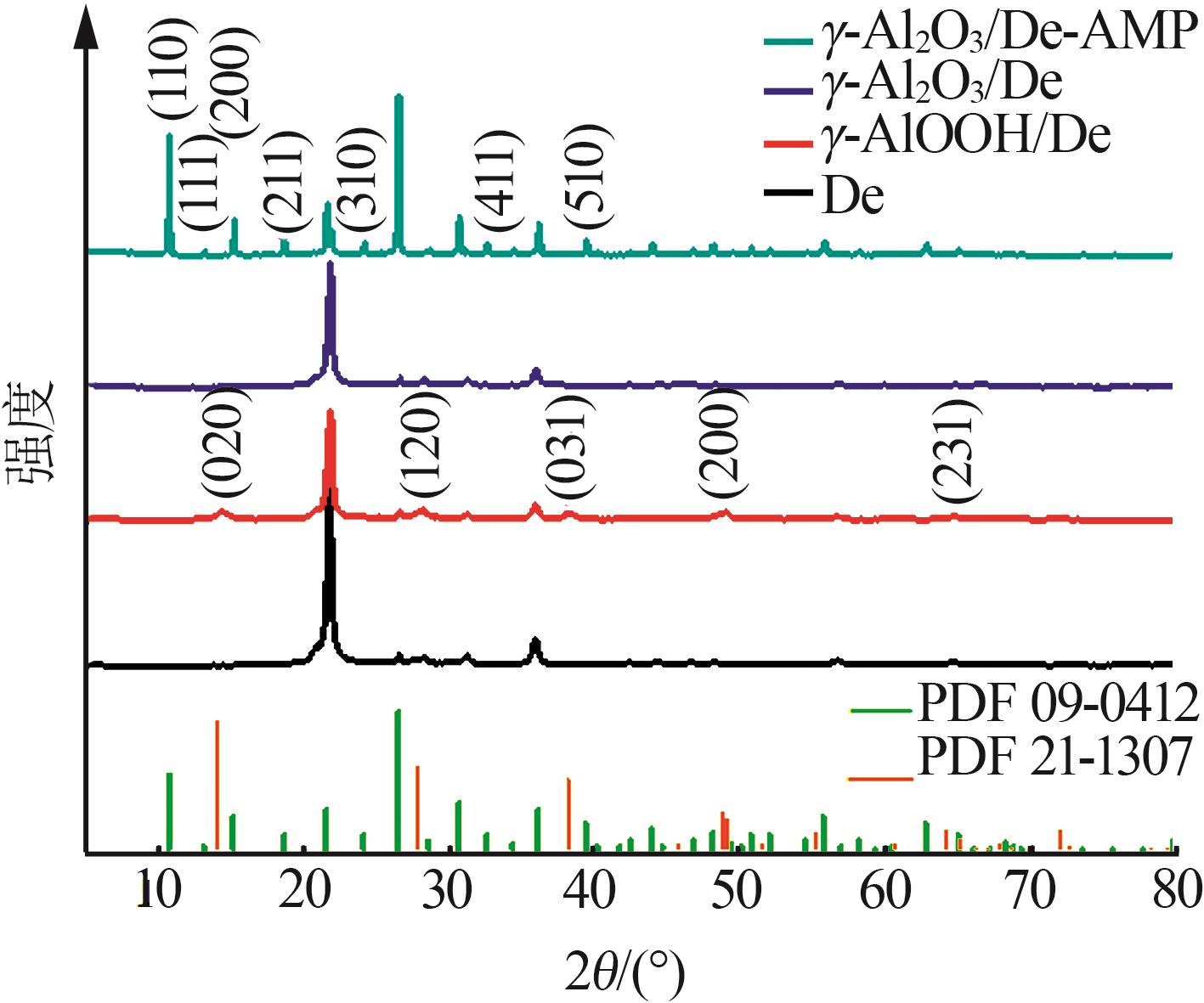
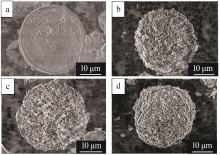
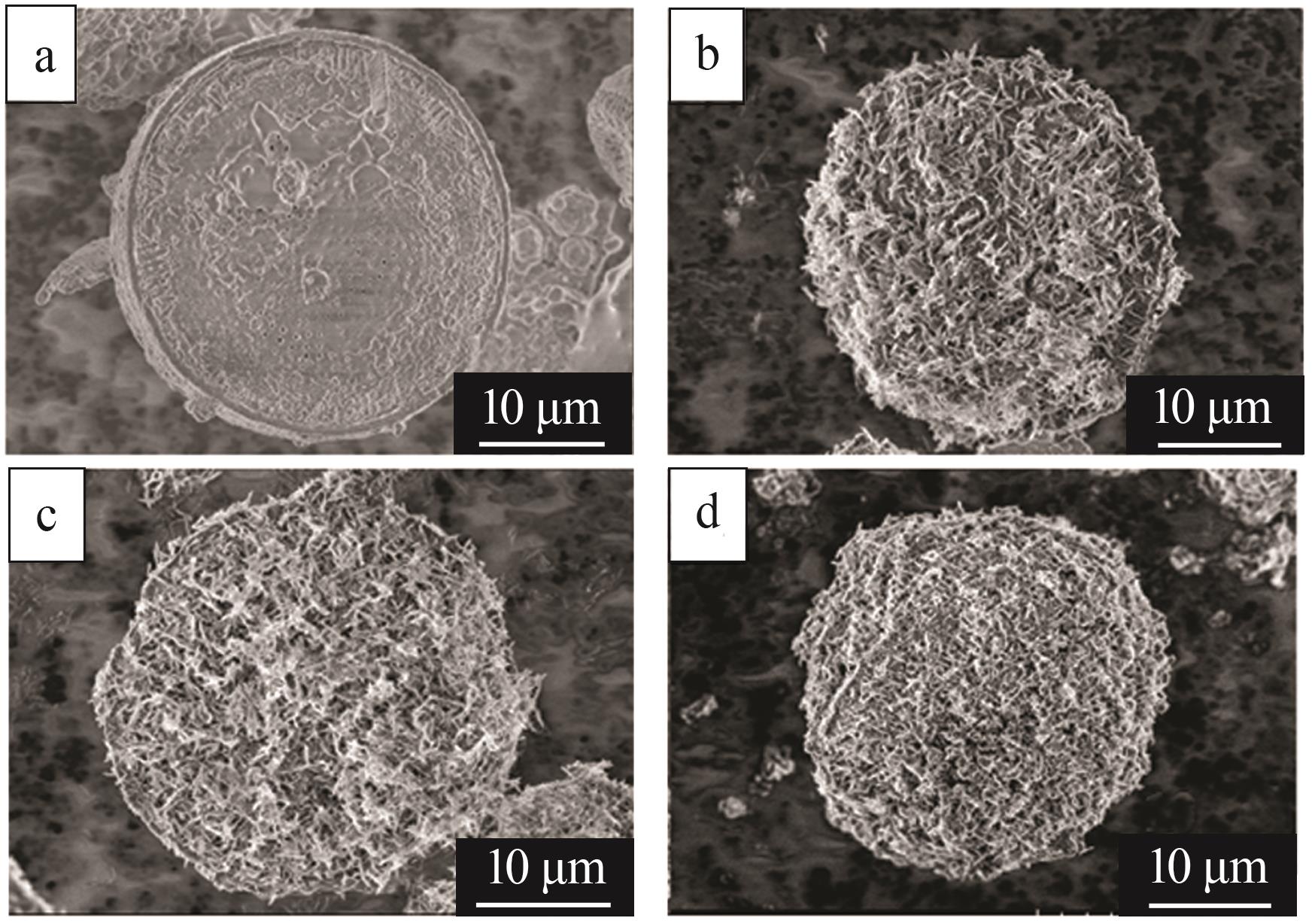
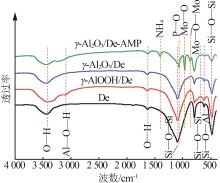
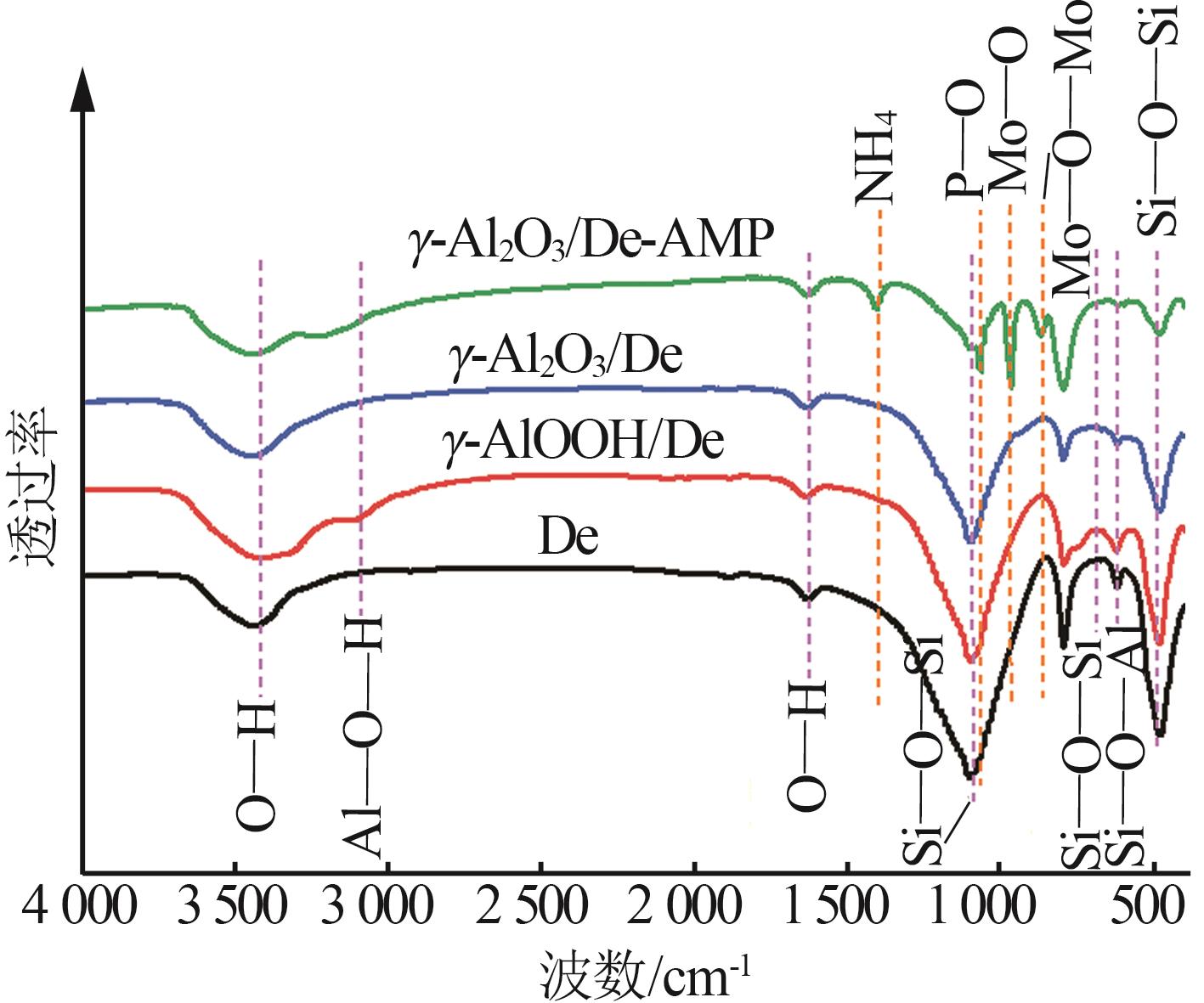
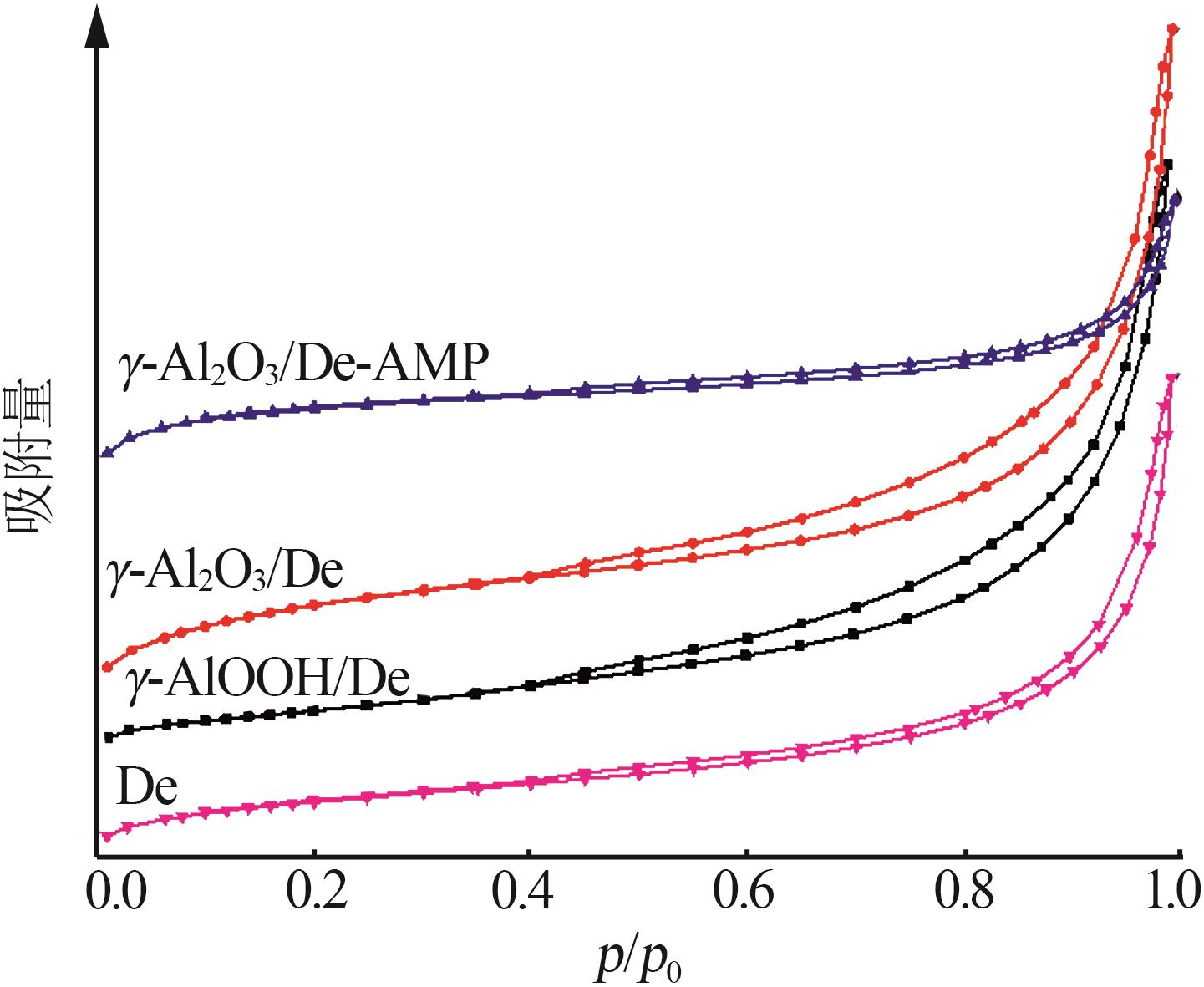
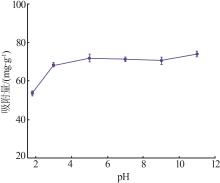
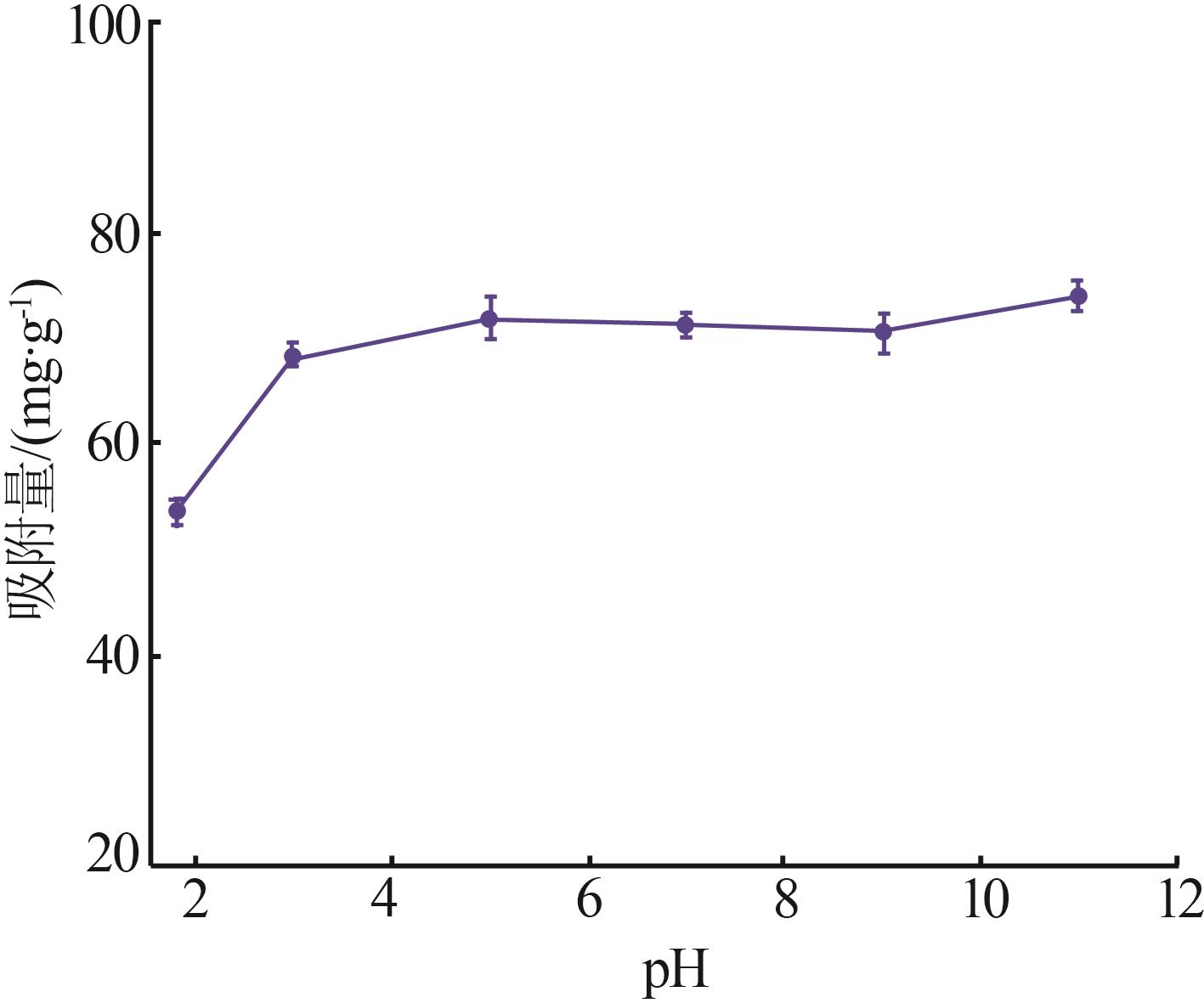
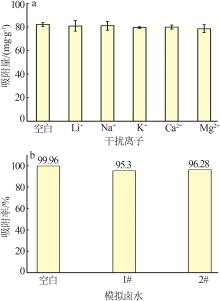
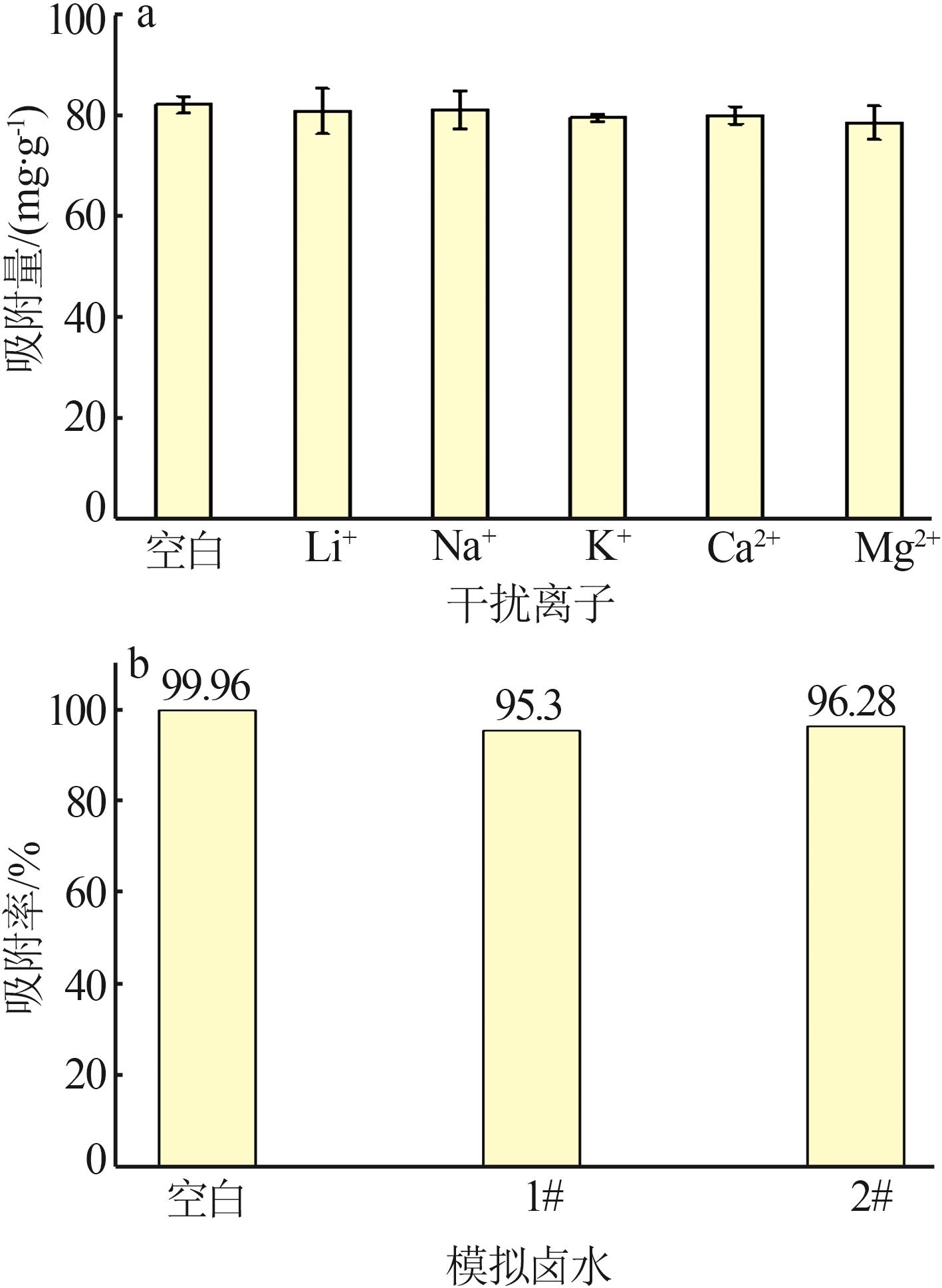
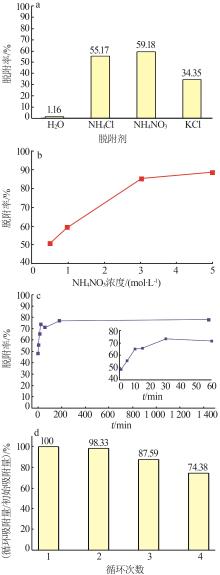
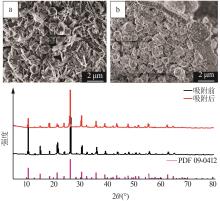
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (11): 19-26.doi: 10.19964/j.issn.1006-4990.2023-0033
• Research & Development • Previous Articles Next Articles
Study on preparation of modified diatomite loaded ammonium phosphomolybdate composite adsorbent and its adsorption performance of Cs+
GUO Xueqin1,2,3( ), DENG Xiaochuan1,2, ZHU Chaoliang1,2, FU Xin4, WANG Ruirui1,2,3, MA Wanxia1,2, FAN Jie1,2, ZUO Fangtao1,2, QING Binju1,2(
), DENG Xiaochuan1,2, ZHU Chaoliang1,2, FU Xin4, WANG Ruirui1,2,3, MA Wanxia1,2, FAN Jie1,2, ZUO Fangtao1,2, QING Binju1,2( )
)
- 1.Key Laboratory of Comprehensive and Highly Efficient Utilization of Salt Lake Resources,Qinghai Institute of Salt Lakes,Chinese Academy of Sciences,Xining 810008,China
2.Qinghai Engineering and Technology Research Center of Comprehensive Utilization of Salt Lake Resources,Xining 810008,China
3.University of Chinese Academy of Sciences,Beijing 100049,China
4.Hangzhou Water Treatment Technology Development Center,Hangzhou 310012,China
-
Received:2023-01-12Online:2023-11-10Published:2023-11-16 -
Contact:QING Binju E-mail:gxq901214@163.com;qbj@isl.ac.cn
CLC Number:
Cite this article
GUO Xueqin, DENG Xiaochuan, ZHU Chaoliang, FU Xin, WANG Ruirui, MA Wanxia, FAN Jie, ZUO Fangtao, QING Binju. Study on preparation of modified diatomite loaded ammonium phosphomolybdate composite adsorbent and its adsorption performance of Cs+[J]. Inorganic Chemicals Industry, 2023, 55(11): 19-26.
share this article
Table 5
Cs+ adsorption properties of AMP-based adsorbents reported in recent years"
| 吸附剂 | qmax/ (mg·g-1) | 吸附平衡时间 | pH |
|---|---|---|---|
| NH4PMA/SBA-15[ | 70.9 | — | — |
| AMP-SiO2[ | 18.13~81.36 | — | — |
| SM-AMP20[ | 37.63 | 4 h | — |
| APS-4[ | 71.28 | 12 h | 2~10 |
| AMP-PA[ | 4.7 | 5 h | 5~10 |
| 50%AMP/P-SBA-15[ | 45.1 | 30 min | 2~9 |
| MCC@AMP[ | 9.49 | 45 min | 2~12 |
| 63.45 | 2 h | 4~11 | |
| AMP@MOF-76(Sm)[ | 57.68 | 50 min | 4~10 |
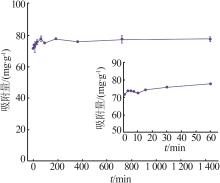
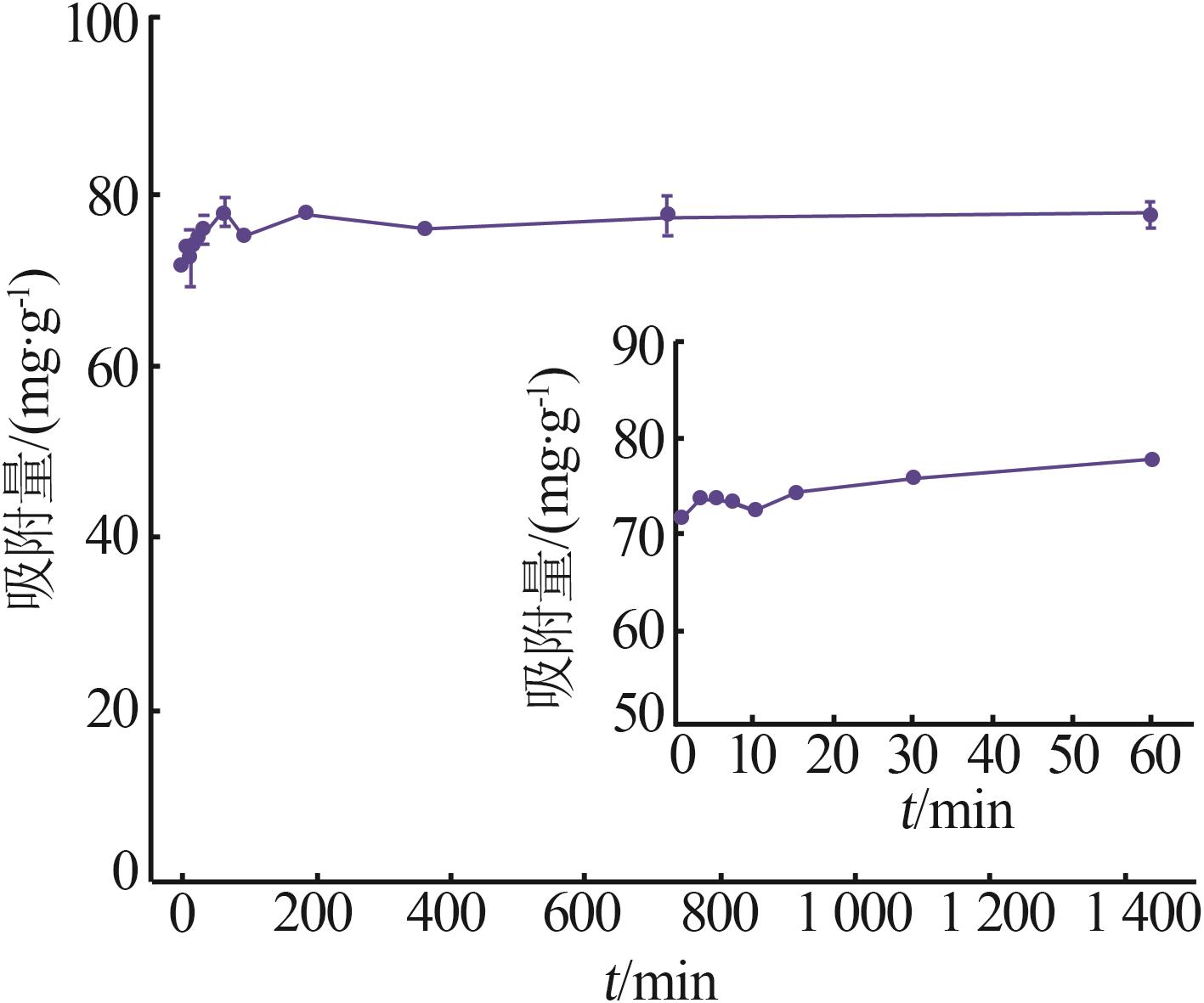
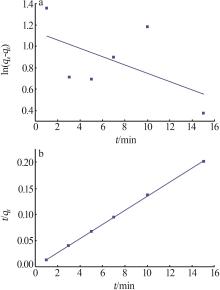
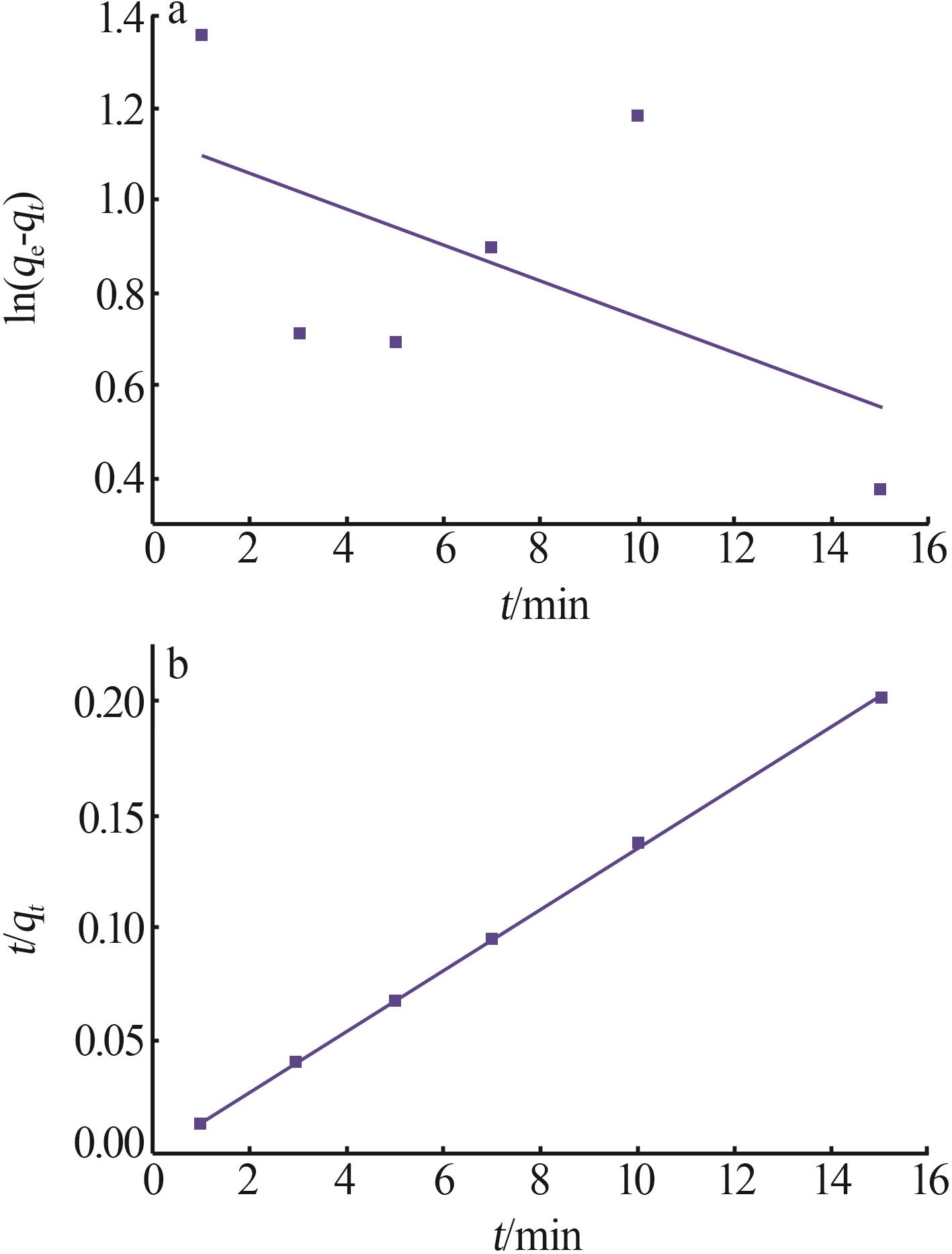
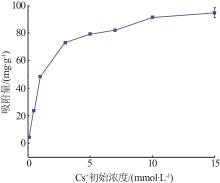
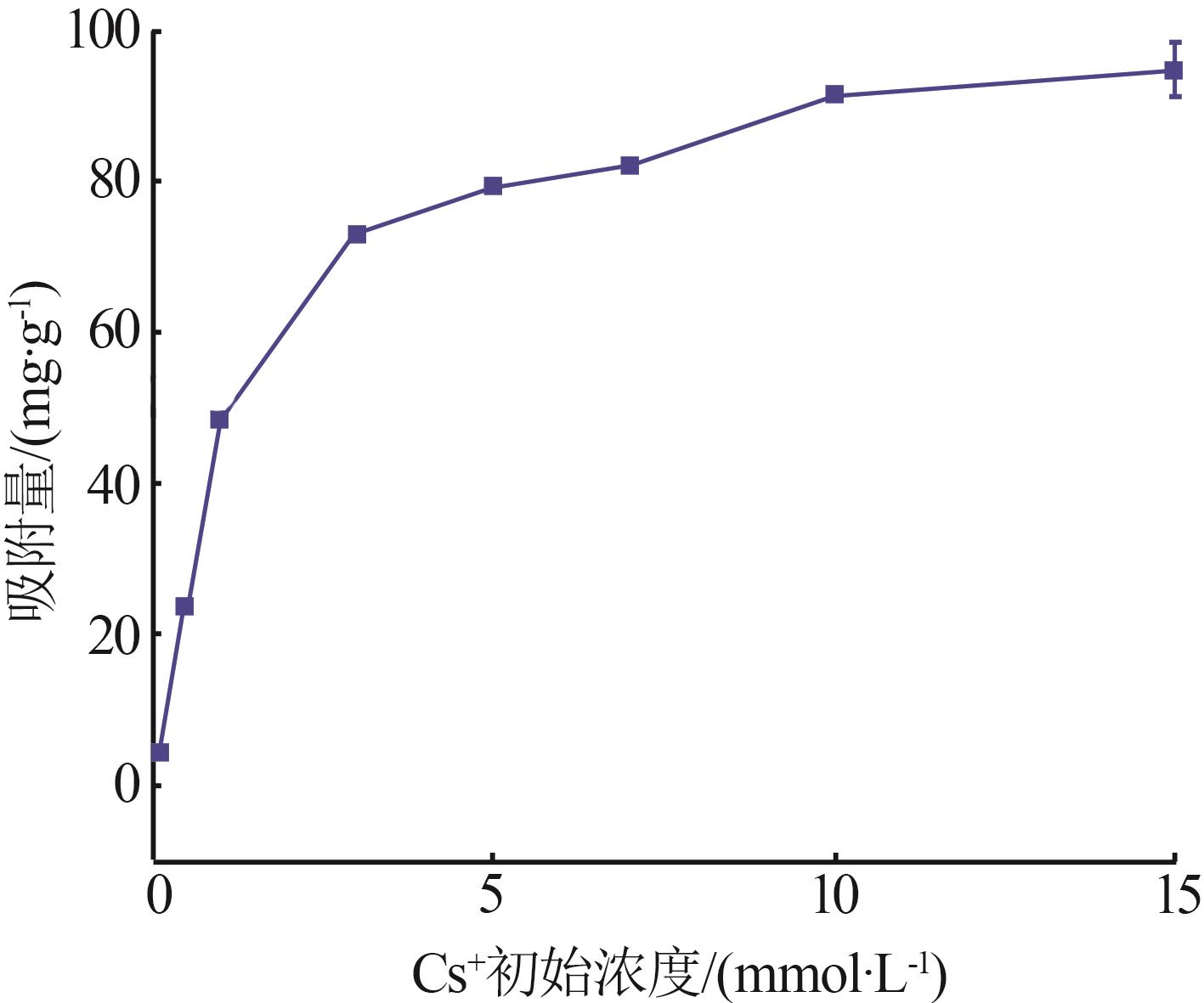
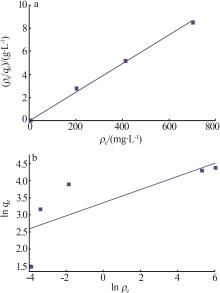
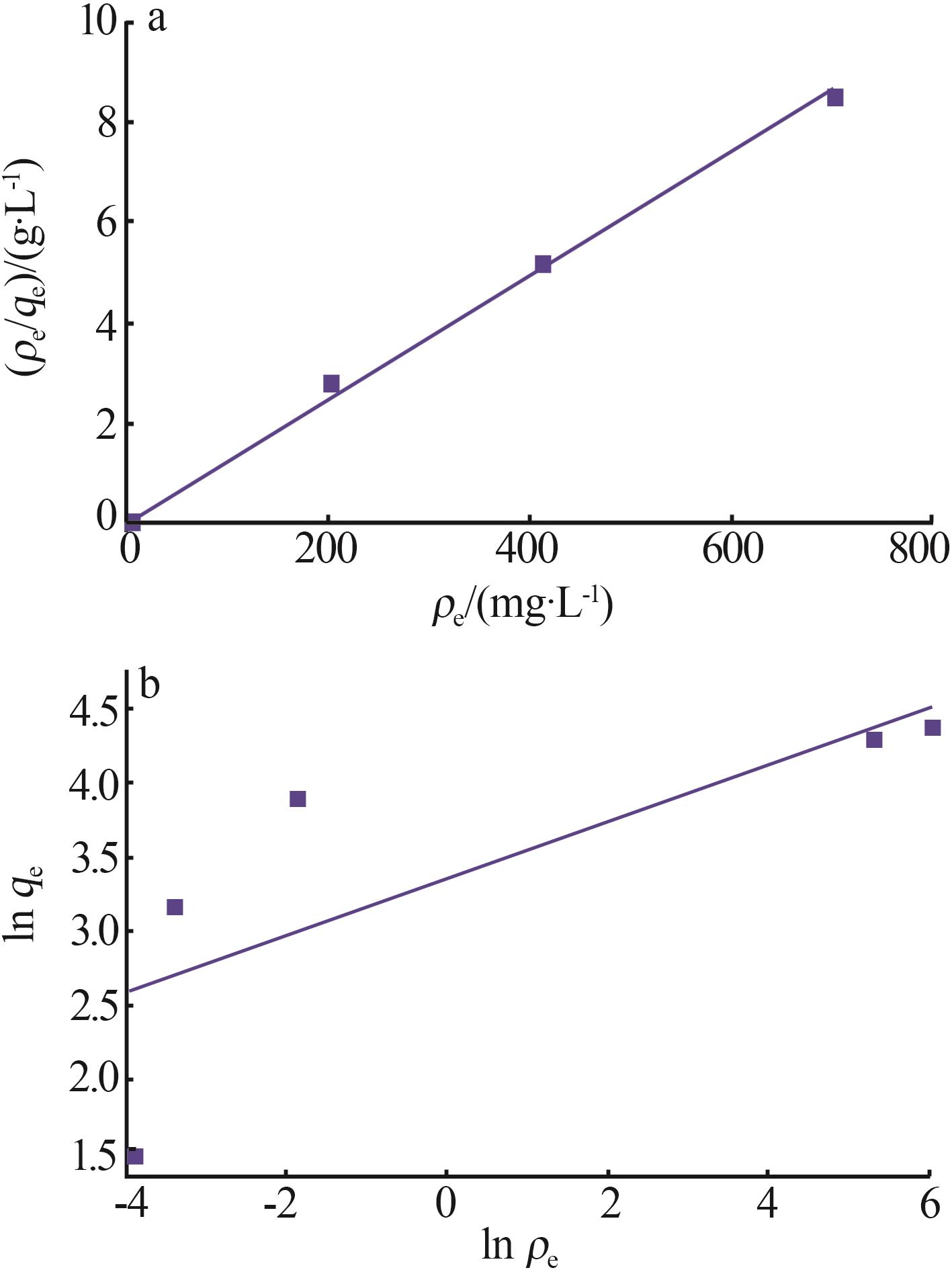
| γ-Al2O3/De-AMP(本研究) | 79.41 | 15 min | 2~11 |
| 1 | 董普,肖荣阁.铯盐应用及铯(碱金属)矿产资源评价[J].中国矿业,2005,14(2):30-34. |
| DONG Pu, XIAO Rongge.Caesium appication and caesium(alkali metals) resource evaluation[J].China Mining Magazine,2005,14(2):30-34. | |
| 2 | 刘力.铷铯发展与思考[J].新疆有色金属,2013,36(6):46-50. |
| LIU Li.Development and thinking of rubidium and cesium[J].Xinjiang Youse Jinshu,2013,36(6):46-50. | |
| 3 | LI Chiping, KANG C Y, HUANG Shuling,et al.Near infrared radiation shielding using Cs x WO3 nanoparticles for infrared mini light-emitting diodes[J].Materials Letters,2020,260:126961. |
| 4 | ZHANG Zelong, SHEN Linli, ZHAO Yi,et al.Coexisting CsPbCl3:CsPbI3 perovskite nanocrystal glasses with high luminescence and stability[J].Chemical Engineering Journal,2020,385:123415. |
| 5 | 郑喜玉,张明刚,徐昶,等.中国盐湖志[M].北京:科学出版社,2002. |
| 6 | 曹文虎,吴蝉.卤水资源及其综合利用技术[M].北京:地质出版社,2004. |
| 7 | 王云生,郑绵平,卜令忠,等.溶液体系中铯分离提取技术的研究进展[J].盐业与化工,2011,40(2):34-37. |
| WANG Yunsheng, ZHENG Mianping, BU Lingzhong,et al.Progress on cesium separation and recovery from the salt lake brines[J].Journal of Salt and Chemical Industry,2011,40(2):34-37. | |
| 8 | CHEN Shangqing, HU Jiayin, HAN Senjian,et al.A review on emerging composite materials for cesium adsorption and environmental remediation on the latest decade[J].Separation and Purification Technology,2020,251:117340. |
| 9 | WANG Xuekai, WANG Jinshu, TENG Weili,et al.Fabrication of highly efficient magnesium silicate and its adsorption behavior towards Cr(Ⅵ)[J].Microporous and Mesoporous Materials,2021,323:111196. |
| 10 | 杜玉成,李生辉,谷恒学,等.巯基/羧基修饰硅藻土及其对Pb(Ⅱ)、Cd(Ⅱ)的吸附性能[J].无机化学学报,2021,37(1):65-73. |
| DU Yucheng, LI Shenghui, GU Hengxue,et al.Mercapto/carboxyl modified diatomite:Adsorption properties to Pb(Ⅱ) and Cd(Ⅱ)[J].Chinese Journal of Inorganic Chemistry,2021,37(1):65-73. | |
| 11 | 靳翠鑫,杜玉成,吴俊书,等.硅藻土原位负载网状纳米结构硅酸镁及其对Cr(Ⅵ)吸附性能[J].无机化学学报,2019,35(4):621-628. |
| JIN Cuixin, DU Yucheng, WU Junshu,et al.In-situ growing nanostructured magnesium silicate on diatomite:Adsorption properties of Cr(Ⅵ)[J].Chinese Journal of Inorganic Chemistry,2019,35(4):621-628. | |
| 12 | DU Yucheng, ZHEN Shuang, WANG Jinshu,et al.FeOOH-MnO2/Sepiolite and Fe2O3-MnO2/Diatomite:Highly efficient adsorbents for the removal of As(Ⅴ)[J].Applied Clay Science,2022,222:106491. |
| 13 | 郑广伟,杜玉成,侯瑞琴,等. γ-AlOOH/Al2O3修饰硅藻土的制备与Cs+、Pb2+吸附性能[J].无机化学学报,2015,31(5):930-938. |
| ZHENG Guangwei, DU Yucheng, HOU Ruiqin,et al.Fabrication and highly efficient adsorption for Cs+ and Pb2+ of γ-AlOOH/Al2O3 modified diatomite[J].Chinese Journal of Inorganic Chemistry,2015,31(5):930-938. | |
| 14 | SCHNEIDER S, GARCEZ A C, TREMBLAY M,et al.Nanoporous ammonium molybdophosphate-silica hybrids as regenerable ultra-selective extraction agents for radiocesium monitoring[J].New Journal of Chemistry,2013,37(12):3877-3880. |
| 15 | CHEN Shangqing, HU Jiayin, SHI Jian,et al.Composite hydrogel particles encapsulated ammonium molybdophosphate for efficiently cesium selective removal and enrichment from wastewat-er[J].Journal of Hazardous Materials,2019,371:694-704. |
| 16 | PARK Y, SHIN W S, CHOI S J.Ammonium salt of heteropoly acid immobilized on mesoporous silica(SBA-15):An efficient ion exchanger for cesium ion[J].Chemical Engineering Journal,2013,220:204-213. |
| 17 | ZHANG Xiaoxia, WU Yan, CHEN Baocai,et al.The effect of γ-ray irradiation on the adsorption properties and chemical stability of AMP/SiO2 towards Cs(I) in HNO3 solution[J].Journal of Radioanalytical and Nuclear Chemistry,2016,310(2):905-910. |
| 18 | 董超超,邓小川,王斌,等.以硅藻土为原料制备铯吸附剂及其吸附性能初探[J].无机盐工业,2021,53(6):134-139. |
| DONG Chaochao, DENG Xiaochuan, WANG Bin,et al.Preparation of cesium adsorbent from diatomite and its adsorption properties[J].Inorganic Chemicals Industry,2021,53(6):134-139. | |
| 19 | WANG Zhijie, DU Hong, GONG Jinghua,et al.Facile synthesis of hierarchical flower-like γ-AlOOH films via hydrothermal route on quartz surface[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2014,450:76-82. |
| 20 | INGALE S V,RAM R, SASTRY P U,et al.Synthesis and characterization of ammonium molybdophosphate-silica nano-composite(AMP-SiO2) as a prospective sorbent for the separation of 137Cs from nuclear waste[J].Journal of Radioanalytical and Nuclear Chemistry,2014,301(2):409-415. |
| 21 | DENG Hao, LI Yuxiang, HUANG Yi,et al.An efficient composite ion exchanger of silica matrix impregnated with ammonium molybdophosphate for cesium uptake from aqueous solution[J].Chemical Engineering Journal,2016,286:25-35. |
| 22 | 胡佩卓,刘莲,王海静,等.磷钼酸铵/聚丙烯酸复合凝胶吸附剂的合成及对铯的分离[J].核化学与放射化学,2019,41(4):361-369. |
| HU Peizhuo, LIU Lian, WANG Haijing,et al.Synthesis of ammonium phosphomolybdate/polyacrylic acid composite gel adsorbent and separation of cesium[J].Journal of Nuclear and Radiochemistry,2019,41(4):361-369. | |
| 23 | DAN Hui, XIAN Qiang, CHEN Luyu,et al.Fabrication of AMP/SBA-15 with various morphologies for cesium removal from aqueous solution[J].Journal of Sol-Gel Science and Technology,2019,91(1):165-177. |
| 24 | DONG Zhen, DU Jifu, CHEN Yanliang,et al.A comparative study of immobilizing ammonium molybdophosphate onto cellulose microsphere by radiation post-grafting and hybrid grafting for cesium removal[J].Environmental Pollution,2021,273:116432. |
| 25 | WANG Yanping, LI Kexin, FANG Dezhen,et al.Ammonium molybdophosphate/metal-organic framework composite as an effective adsorbent for capture of Rb+ and Cs+ from aqueous solution[J].Journal of Solid State Chemistry,2022,306:122767. |
| [1] | BAI Xingxing, LI Hanfei, TANG Yong, ZHANG Jun, ZHU Guangkai, LI Lishuo, TONG Zhangfa. Study on preparation of cellulose based hydrogel doped with nano-calcium carbonate and its adsorption properties of copper ions [J]. Inorganic Chemicals Industry, 2025, 57(2): 83-91. |
| [2] | ZHANG Yanyan, LI Donghong, LIU Yonghe, ZHANG Yang, KANG Le, WANG Yi. Study on surface treatment of alumina filler [J]. Inorganic Chemicals Industry, 2025, 57(1): 77-82. |
| [3] | TIAN Peng, ZHANG Haoran, XU Jingang, MOU Chenxi, XU Qianjin, NING Guiling. Study on aluminum sol modified anode and cathode materials for lithium ion batteries [J]. Inorganic Chemicals Industry, 2024, 56(9): 44-53. |
| [4] | WANG Ping, XU Rongsheng, SUN Dong, SHI Xiaohong, XU Wei, LI Mei. Study on preparation of nitrogen-doped biochar and its adsorption properties for methylene blue [J]. Inorganic Chemicals Industry, 2024, 56(9): 117-127. |
| [5] | SONG Zhaoxia, LIU Yongkang, GUO Yaokun, WANG Tengfei. Study on adsorption performance of wheat straw biochar on malachite green [J]. Inorganic Chemicals Industry, 2024, 56(9): 128-135. |
| [6] | XIONG Chengrong, CHEN Yan, TANG Miao, ZHANG Fengze, ZHOU Tianxiang, OUYANG Ruifeng, DONG Gang, SHI Wei, ZENG Tao, CHEN Yunxia, SU Xiaoli. Research progress on preparation of 2D vermiculite nanosheets and their environmental adsorption of pollutants [J]. Inorganic Chemicals Industry, 2024, 56(8): 19-26. |
| [7] | ZHANG Bangcheng, WANG Li. Preparation and adsorption properties of waste polyester⁃based activated carbon activated by ZnCl2 [J]. Inorganic Chemicals Industry, 2024, 56(7): 126-134. |
| [8] | ZHANG Lijin, LÜ Qing, CHEN Xiaolang, LI Qingxin, SHI Hongyu, QIN Jun. Preparation of Ca-based LDO composite material and its adsorption performance for phosphate [J]. Inorganic Chemicals Industry, 2024, 56(7): 37-45. |
| [9] | ZHAO Chuang, ZHANG Boyu, LI Ben, JIN Fengying, LI Bin, SUN Zhenhai, GUO Chunlei. Study on adsorption and separation technology of polycyclic aromatic hydrocarbons by adsorbent [J]. Inorganic Chemicals Industry, 2024, 56(7): 61-68. |
| [10] | REN Xuechang, FENG Hao. Study on dissolution process of sintered clinker by aluminum ash alkali method with ultrasound irritation [J]. Inorganic Chemicals Industry, 2024, 56(6): 119-126. |
| [11] | LIU Kailong, ZHU Kongyi, GUO Chunlei, MA Xiaobiao, WANG Yujian, SHENG Qiang, LI Xiang, WANG Yinbin, JIN Fengying. Effects of process condition on performance of diesel aromatic to light aromatic [J]. Inorganic Chemicals Industry, 2024, 56(6): 139-146. |
| [12] | ZHU Zenghu, WANG Min, PENG Zhengjun, JIA Guofeng, LI Yan. Study on adsorption of potassium ions in lithium chloride solution [J]. Inorganic Chemicals Industry, 2024, 56(6): 61-66. |
| [13] | XU Mengyao, ZHANG Xin, HE Kunpeng, HE Jian, JIANG Wei. Preparation of yttrium oxide and zirconium phosphate adsorbents from zirconium-yttrium waste and evaluation of their performance [J]. Inorganic Chemicals Industry, 2024, 56(3): 116-124. |
| [14] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [15] | LI Yang, LOU Feijian, SUI Xin, LI Keyan, LIU Fei, GUO Xinwen. Preparation of amine-functionalized fumed SiO2 materials and their performance for CO2 adsorption [J]. Inorganic Chemicals Industry, 2024, 56(2): 38-43. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||