Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (10): 141-148.doi: 10.19964/j.issn.1006-4990.2022-0306
• Catalytic Materials • Previous Articles Next Articles
Effect of Ni on selective hydrodegenation of polyaromatic hydrocarbons of Pt/γ-Al2O3 catalyst
JIN Fengying1,2( ),YU Haibin1,WANG Yaquan2,ZANG Jiazhong1,GUO Chunlei1,LIU Hang1,MA Mingchao1,ZHAO Xunzhi1,LIU Kailong1
),YU Haibin1,WANG Yaquan2,ZANG Jiazhong1,GUO Chunlei1,LIU Hang1,MA Mingchao1,ZHAO Xunzhi1,LIU Kailong1
- 1. CenerTech Tianjin Chemical Research and Design Institute Co. , Ltd. , Tianjin 300131, China
2. Institute of Chemical Engineering, Tianjin University
-
Received:2022-05-18Online:2022-10-10Published:2022-11-03
CLC Number:
Cite this article
JIN Fengying,YU Haibin,WANG Yaquan,ZANG Jiazhong,GUO Chunlei,LIU Hang,MA Mingchao,ZHAO Xunzhi,LIU Kailong. Effect of Ni on selective hydrodegenation of polyaromatic hydrocarbons of Pt/γ-Al2O3 catalyst[J]. Inorganic Chemicals Industry, 2022, 54(10): 141-148.
share this article
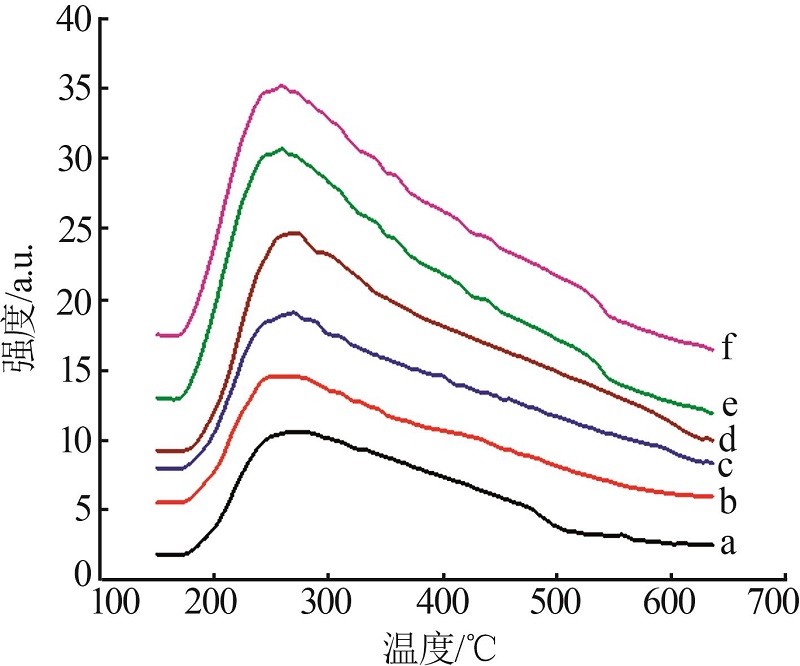
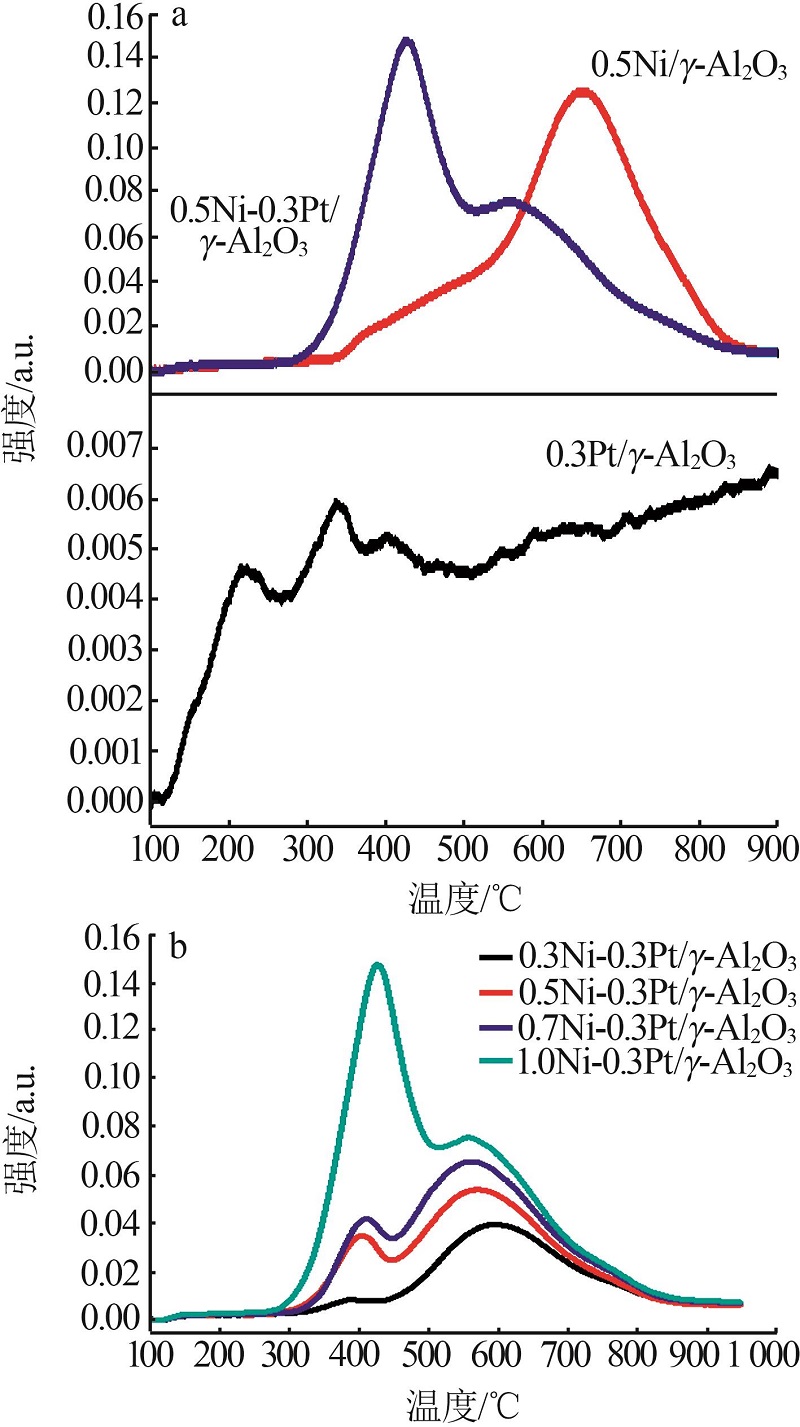
Table 1
Characterization results of catalysts by N2 adsorption-desorption and ICP-AES"
| 编号 | 样品 | ICP表征 | N2物理吸附 | |||
|---|---|---|---|---|---|---|
w (Ni)/% | w (Pt)/% | SBET/ (m2?g-1) | VT/ (cm3?g-1) | |||
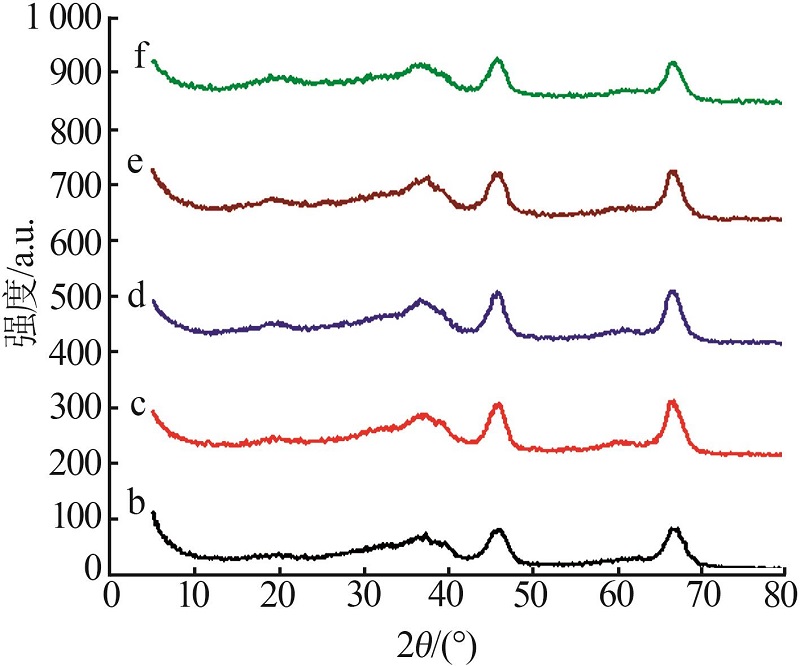
| a | γ-Al2O3 | — | — | 401.20 | 0.75 | |
| b | 0.3Pt/γ-Al2O3 | — | 0.300 | 389.10 | 0.73 | |
| c | 0.3Ni-0.3Pt/ γ-Al2O3 | 0.298 | 0.301 | 375.69 | 0.72 | |
| d | 0.5Ni-0.3 Pt/ γ-Al2O3 | 0.501 | 0.299 | 372.17 | 0.71 | |
| e | 0.7Ni-0.3 Pt/ γ-Al2O3 | 0.699 | 0.299 | 371.17 | 0.70 | |
| f | 1.0Ni-0.3 Pt/ γ-Al2O3 | 1.001 | 0.301 | 370.09 | 0.69 | |
| g | 0.5Ni-0.2 Pt/ γ-Al2O3 | 0.499 | 0.199 | 373.58 | 0.70 | |
| h | 0.5Ni-0.1 Pt/ γ-Al2O3 | 0.502 | 0.101 | 379.32 | 0.70 | |
Table 2
NH3-TPD characterization results of different samples"
| 项目 | 样品 | 酸量/ (mmol?g-1) | 酸强度/ ℃ |
|---|---|---|---|
| a | γ-Al2O3 | 0.18 | 245.1 |
| b | 0.3Pt/γ-Al2O3 | 0.20 | 246.3 |
| c | 0.3Ni-0.3Pt/γ-Al2O3 | 0.23 | 245.1 |
| d | 0.5Ni-0.3 Pt/γ-Al2O3 | 0.25 | 247.8 |
| e | 0.7Ni-0.3 Pt/γ-Al2O3 | 0.28 | 247.6 |
| f | 1.0Ni-0.3 Pt/γ-Al2O3 | 0.27 | 248.2 |
| g | 0.5Ni-0.2 Pt/γ-Al2O3 | 0.24 | 247.9 |
| h | 0.5Ni-0.1 Pt/γ-Al2O3 | 0.23 | 247.7 |
Table 5
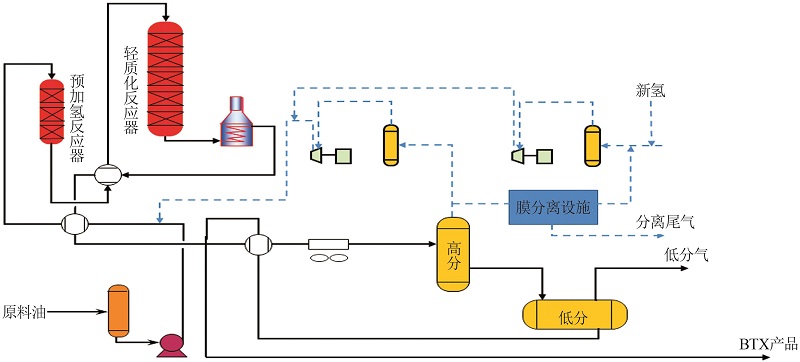
Effect of PAHs contents on composition of lightweight gas phase products"
| 项目 | 原料组成质量分数/% | 产品组成质量分数/% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 稠环芳烃 | 单环芳烃 | 甲烷 | 乙烷 | 丙烷 | 丙烯 | 异丁烷 | 正丁烷 | 丁烯 | 异戊烷 | 正戊烷 | ||
| C10+重芳烃 | 52.8 | 46.7 | 2.4 | 30.6 | 47.3 | 2.1 | 9.0 | 4.4 | 2.7 | 1.2 | 0.3 | |
| 加氢原料1 | 32.5 | 61.2 | 1.8 | 34.4 | 45.9 | 0.0 | 10.5 | 6.6 | 0.1 | 0.0 | 0.7 | |
| 加氢原料2 | 25.0 | 66.6 | 2.0 | 36.9 | 44.1 | 0.2 | 9.9 | 6.3 | 0.3 | 0.0 | 0.3 | |
| 加氢原料3 | 18.0 | 71.6 | 1.8 | 39.3 | 42.9 | 0.2 | 8.7 | 5.0 | 0.7 | 1.1 | 0.3 | |
| 加氢原料4 | 13.8 | 74.6 | 1.8 | 44.6 | 38.9 | 0.5 | 7.5 | 4.6 | 0.8 | 0.4 | 0.9 | |
Table 6
Effect of PAHs contents on composition of lightweight liquid phase products"
| 项目 | w(稠环芳烃) | w(C5+非芳烃) | w(C6芳烃) | w(C7芳烃) | w(C8芳烃) | w(C9芳烃) | w(C10芳烃) | w(C10+芳烃) |
|---|---|---|---|---|---|---|---|---|
| C10+重芳烃 | 53.5 | 7.7 | 9.2 | 23.5 | 23.9 | 10.8 | 4.7 | 20.2 |
| 预加氢产品1 | 32.5 | 8.0 | 9.9 | 26.1 | 18.6 | 10.9 | 4.4 | 12.1 |
| 预加氢产品2 | 25.0 | 8.3 | 10.7 | 26.9 | 30.5 | 11.8 | 4.1 | 7.7 |
| 预加氢产品3 | 18.0 | 8.5 | 10.8 | 28.4 | 33.0 | 12.6 | 3.6 | 3.1 |
| 预加氢产品4 | 13.8 | 8.7 | 10.7 | 28.8 | 34.3 | 11.7 | 3.5 | 2.3 |
| [1] | 赵仁殿, 金彰礼, 陶志华, 等. 芳烃工学[M].北京:化学工业出版社, 2001: 251-256. |
| ZHAO Rendian, JIN Zhangli, TAO Zhihua, et al.Aromatics engineering[M].Beijing:Chemical Industry Press(CIP), 2001: 251-256 | |
| [2] | 孔德金, 祁晓岚, 朱志荣, 等. 重芳烃轻质化技术进展[J].化工进展, 2006, 25(9): 983-987. |
| KONG Dejin, QI Xiaolan, ZHU Zhirong, et al.Technological advances in conversion of heavy aromatics to light aromatics[J].Chemical Industry and Engineering Progress, 2006, 25(9): 983-987 | |
| [3] | 臧甲忠, 郭春垒, 范景新, 等. C9 +重芳烃增产BTX技术进展[J].化工进展, 2017, 36(4): 1278-1287. |
| ZANG Jiazhong, GUO Chunlei, FAN Jingxin, et al.Advance in BTX production increase technology from C9 + heavy aromatics[J].Chemical Industry and Engineering Progress, 2017, 36(4): 1278-1287. | |
| [4] | 范景新, 臧甲忠, 于海斌, 等. 重芳烃轻质化研究进展[J].工业催化, 2015, 23(9): 666-673. |
| FAN Jingxin, ZANG Jiazhong, YU Haibin, et al.Research progress in conversion of heavy aromatics to light ones[J].Industrial Catalysis, 2015, 23(9): 666-673. | |
| [5] | 杨纪, 靳凤英, 范景新, 等. 分子筛重芳烃加氢脱烷基催化剂研究进展[J].无机盐工业, 2017, 49(6): 1-6. |
| YANG Ji, JIN Fengying, FAN Jingxin, et al.Research progress of zeolites in catalysts for hydrodealkylation of heavy aromatics[J].Inorganic Chemicals Industry, 2017, 49(6): 1-6. | |
| [6] | 许杰, 尹宏峰.重芳烃油加氢精制催化剂及重芳烃油生产BTX的方法: 中国,114433119A[P].2022-05-06. |
| XU Jie, YIN Hongfeng.A method for preparing hydrofining catalysts and producing BTX of heavy aromatic oil:CN,114433119A[P].2022-05-06. | |
| [7] | 李水荣, 徐保岳, 楼巧琳.一种重芳烃油加氢精制催化剂及重芳烃油加工方法: 中国,114433111A[P].2022-05-06. |
| LI Shuirong, XU Baoyue, LOU Qiaolin.A method for preparing hydrofining catalysts and conversion of heavy aromatic hydrocarbons:CN,114433111A[P].2022-05-06. | |
| [8] | 刘航, 臧甲忠, 范景新, 等. 重芳烃轻质化催化剂n(Ni)/n(Ni+Mo)的优化与分析[J].无机盐工业, 2021, 53(12): 140-145. |
| LIU Hang, ZANG Jiazhong, FAN Jingxin, et al.Optimization and analysis of n(Ni)/n(Ni+Mo) of heavy aromatics to light aromatics catalyst[J].Inorganic Chemicals Industry, 2021, 53(12): 140-145 | |
| [9] | 陈庆龄, 孔德金, 杨卫胜.对二甲苯增产技术发展趋向[J].石油化工, 2004, 33(10): 909-915. |
| CHEN Qingling, KONG Dejin, YANG Weisheng.Developmental trends in p-xylene production increasing technology[J].Petrochemical Technology, 2004, 33(10): 909-915. | |
| [10] | 祁晓岚, 左煜, 陈雪梅, 等. HAT-plus重芳烃轻质化技术[J].石油学报:石油加工, 2008, 24(S1): 338-341. |
| QI Xiaolan, ZUO Yu, CHEN Xuemei, et al.HAT-plus technology for conversion of heavy aromatics to light aromatics[J].Acta Petrolei Sinica:Petroleum Processing Section, 2008, 24(S1): 338-341. | |
| [11] | 程文才, 杨德琴, 朱志荣, 等. 用于重质芳烃加氢脱烷基与烷基转移的催化剂:中国,1055959C[P].2000-08-30. |
| CHENG Wencai, YANG Deqin, ZHU Zhirong, et al.Catalysts for hydrodealkylation and alkylation of heavy aromatics:CN,1055959C[P].2000-08-30. | |
| [12] | 李经球, 孔德金, 郭毅.用于重芳烃转化的催化反应系统和催化重芳烃转化的方法:中国,114436736A[P].2022-05-06 |
| LI Jingqiu, KONG Dejin, GUO Yi.Catalytic reaction system and catalysts preparation for conversion of heavy aromatics:CN,114436736A[P].2022-05-06. | |
| [13] | 黄新露.重芳烃高效转化生产轻芳烃技术[J].化工进展, 2013, 32(9): 2263-2266. |
| HUANG Xinlu.Technology for producing light aromatics from heavy aromatics[J].Chemical Industry and Engineering Progress, 2013, 32(9): 2263-2266. | |
| [14] | YASUDA H, YOSHIMURA Y.Hydrogenation of tetralin over zeolite-supported Pd-Pt catalysts in the presence of dibenzothiophene[J].Catalysis Letters, 1997, 46(2): 43-48. |
| [15] | KISHORE KUMAR S A, JOHN M, PAI S M, et al.Low temperature hydrogenation of aromatics over Pt-Pd/SiO2-Al2O3 cataly-st[J].Fuel Processing Technology, 2014, 128: 303-309. |
| [16] | TÉLLEZ-ROMERO J G, CUEVAS-GARCÍA R, RAMÍREZ J, et al.Simultaneous naphthalene and thiophene hydrogenation over Ni(X)-Pt/HMOR catalysts[J].Catalysis Today, 2015, 250: 12-20 |
| [17] | 姬宝艳.镍基催化剂上稠环芳烃选择性加氢的研究[D].西安:西北大学, 2017. |
| JI Baoyan.Selective hydrogenation of polycyclic aromatic hydrocarbons over Ni catalysts[D].Xi'an:Northwest University, 2017. | |
| [18] | PAWELEC B, PAROLA V L, THOMAS S, et al.Enhancement of naphthalene hydrogenation over PtPd/SiO2-Al2O3 catalyst modified by gold[J].Journal of Molecular Catalysis A:Chemical, 2006, 253(1/2): 30-43. |
| [19] | 杨基和, 旷俊杰, 陈玉龙.重质芳烃油选择性加氢工艺研究[J].化学工程, 2012, 40(4): 10-13. |
| YANG Jihe, KUANG Junjie, CHEN Yulong.Studies on selective hydrogenation of heavy aromatic oil[J].Chemical Engineering(China), 2012, 40(4): 10-13. | |
| [20] | 李晓云, 于海斌, 孙彦民, 等. 无机铝盐辅助活性氧化铝水热合成拟薄水铝石[J].无机盐工业, 2016, 48(6): 35-37. |
| LI Xiaoyun, YU Haibin, SUN Yanmin, et al.Inorganic aluminum salt assisted hydrothermal synthesis of pseudoboehmite by activated alumina[J].Inorganic Chemicals Industry, 2016, 48(6): 35-37. | |
| [21] | YANG Renchun, LI Xiaogang, WU Junsheng, et al.Hydrotreating of crude 2-ethylhexanol over Ni/Al2O3 catalysts:Surface Ni species-catalytic activity correlation[J].Applied Catalysis A:General, 2009, 368(1/2): 105-112. |
| [22] | 王丹, 周清华, 王冰, 等. 制备方法对镍系催化剂的影响[J].石油化工, 2015, 44(10): 1193-1198. |
| WANG Dan, ZHOU Qinghua, WANG Bing, et al.Effects of preparation methods on nickel-based catalysts[J].Petrochemical Technology, 2015, 44(10): 1193-1198. | |
| [23] |
IM J, SHIN H, JANG H, et al.Maximizing the catalytic function of hydrogen spillover in platinum-encapsulated aluminosilicates with controlled nanostructures[J].Nature Communications, 2014,29 Doi:10.1038/ncomms4370 .
doi: 10.1038/ncomms4370 |
| [1] | YANG Fu, XIE Yulong. Study on preparation and Na+ doping modification of ternary material LiNi0.65Co0.15Mn0.2O2 [J]. Inorganic Chemicals Industry, 2025, 57(3): 43-49. |
| [2] | ZHOU Xuan, LI Mengrui, CHEN Yichen, FAN Huiqiang, WANG Bin, YUAN Gang. Research progress of nickel-based phosphide composites in improving of catalytic water electrolysis for hydrogen evolution performance [J]. Inorganic Chemicals Industry, 2024, 56(4): 8-15. |
| [3] | WANG Hangyu, DU Yifa, GUO Xia, NA Yuxuan, WAN Macuo, ZHOU Yongquan. Study on preparation of NiCo Prussian blue analogue hollow nanobubbles and their Cs+ adsorption properties [J]. Inorganic Chemicals Industry, 2024, 56(10): 55-63. |
| [4] | LIAO Shuangshuang, XIE Tangfeng, GONG Qinxue, YUAN Guobin. Determination of trace thallium in nickel sulfate by inductively coupled plasma mass spectrometry [J]. Inorganic Chemicals Industry, 2023, 55(9): 121-125. |
| [5] | MA Zhiyuan, LÜ Dawei, WANG Hui, JIN Nannan, ZHU Jinjian, ZHANG Jingcheng. Industrial application of THDS-2/3 catalyst in capacity expansion of hydrofining plant [J]. Inorganic Chemicals Industry, 2023, 55(8): 140-144. |
| [6] | FENG Zhun. Improvement of high temperature stability of high nickel single crystal cathode materials by B/Al/Zr synergistic strategy [J]. Inorganic Chemicals Industry, 2023, 55(8): 59-64. |
| [7] | LI Tong, YIN Hongfeng. Study on controllable preparation of nickel phyllosilicate nanotubes catalysts and their catalytic performance [J]. Inorganic Chemicals Industry, 2023, 55(5): 128-136. |
| [8] | SUN Zhigao, WU Yuan, YANG Xingchun, ZHANG Dongliang, WANG Mitang. Preparation and properties of high-magnesium nickel slag-fly ash based geopolymer [J]. Inorganic Chemicals Industry, 2023, 55(11): 139-146. |
| [9] | YANG Wenlong,CHENG Peng,LU Yanfei,NAN Zhiyu,LI Yuhang,LI Shisong,WANG Chunlei,SHU Chang,ZHANG Dongsheng. Study on preparation and performance of Ni/Al2O3 hydrogenation catalyst [J]. Inorganic Chemicals Industry, 2022, 54(7): 141-148. |
| [10] | DING Yong,FENG Xiaorui,LU Xiangjie,GAO Xiaoqin,FENG Yan,HE Yan. Study on preparation of high purity micron nickel oxide powder by roasting carbonyl nickel powder [J]. Inorganic Chemicals Industry, 2022, 54(4): 119-122. |
| [11] | ZOU Changwu,SUN Jiu,FAN Weifeng,ZHANG Bin,WANG Zhengqiang,ZHANG Zheng. Study on preparation and electrochemical properties of lithium nickel cobalt aluminate modified by boric acid spray coating [J]. Inorganic Chemicals Industry, 2022, 54(1): 66-70. |
| [12] | Zhao Duan,Zhou Geng,Hou Shunli,Lan Ziwei,Zhang Jianru,Li Jian,Wang Jiatai. Research progress of coating modification of high nickel ternary materials for lithium-ion batteries [J]. Inorganic Chemicals Industry, 2021, 53(8): 1-7. |
| [13] | Ma Mingchao,Zang Jiazhong,Yu Haibin,Fan Jingxin,Guo Chunlei,Jin Fengying. Research progress rational design of noble metal catalysts for selective hydrogenation [J]. Inorganic Chemicals Industry, 2021, 53(7): 23-29. |
| [14] | Zhang Shangqiang,Sun Guanhua,Sun Yanmin,Zhu Jinjian,Nan Jun,Xiao Han,Zhang Jingcheng,Song Guoliang. Study on preparation of CNTs-Al2O3 supported Pd catalyst and its performance [J]. Inorganic Chemicals Industry, 2021, 53(6): 194-198. |
| [15] | Hu Xu,Dong Lingyu,Li Wencui,Hao Guangping. Preparation of transition metal-nitrogen co-doped porous carbon-based CO2electro-reduction catalyst through photochemical method [J]. Inorganic Chemicals Industry, 2021, 53(6): 8-13. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||