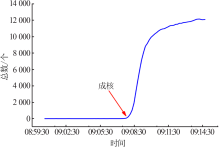
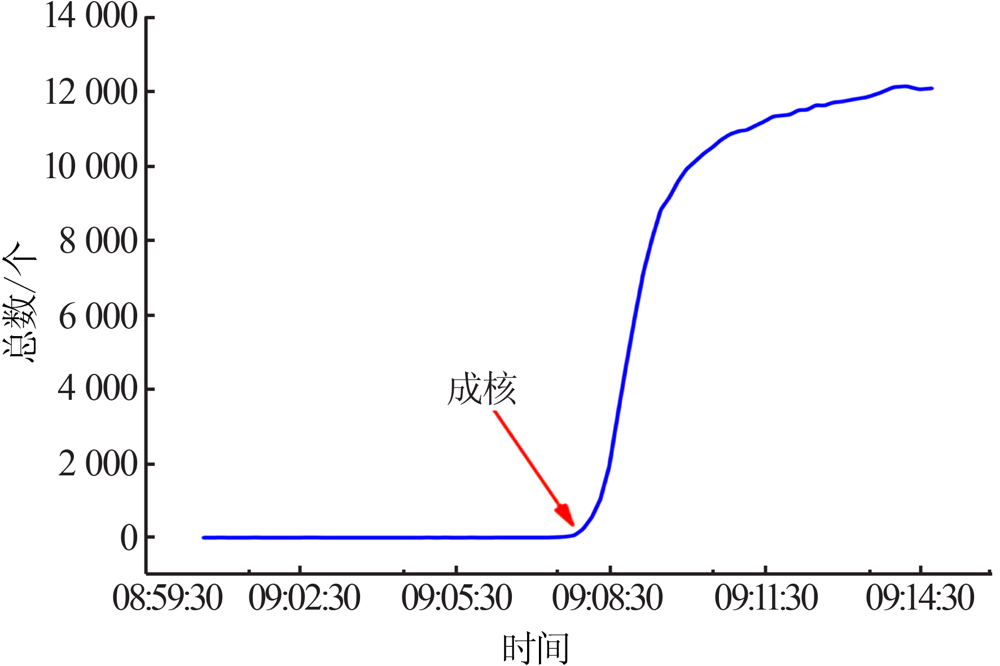
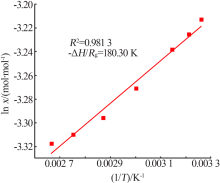
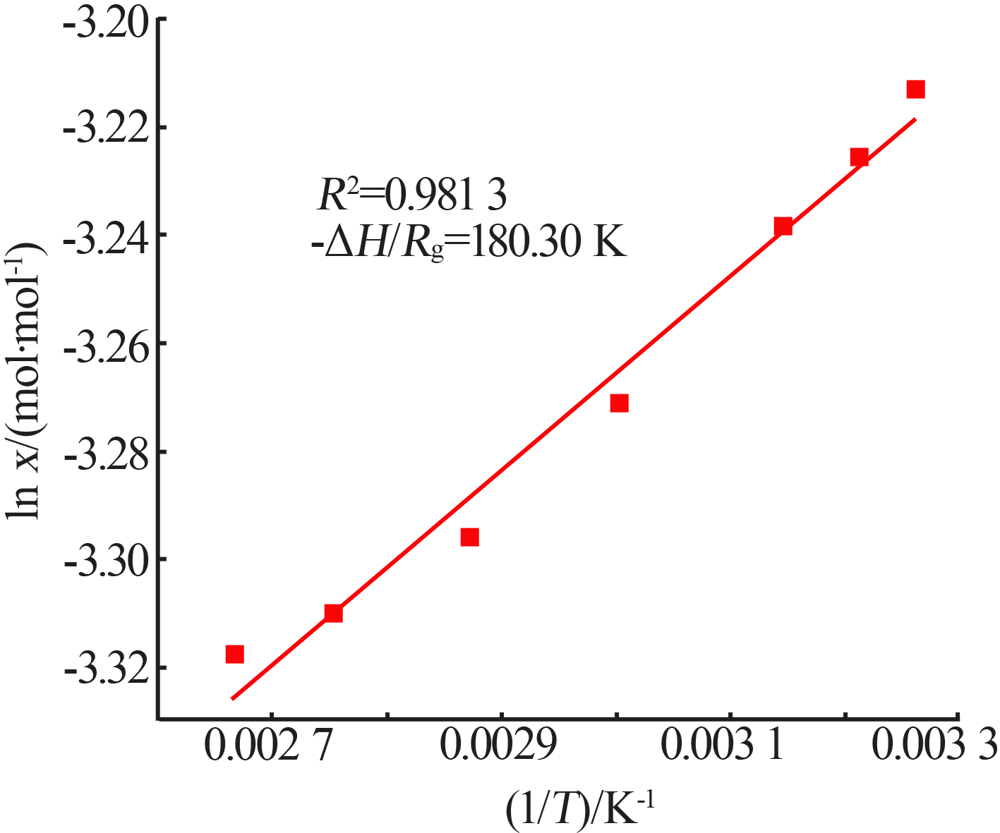
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (10): 41-46.doi: 10.19964/j.issn.1006-4990.2021-0037
• Research & Development • Previous Articles Next Articles
Study on nucleation kinetics of sodium sulfate-water system based on inverse solubility
Wang Yanfei( ),Jiao Jian,Jiang Shuwan,Xu Shijie(
),Jiao Jian,Jiang Shuwan,Xu Shijie( )
)
- Tianjin Key Laboratory of Brine Chemical Engineering and Ecological Utilization of Resources,College of Chemical Engineering and Materials,Tianjin University of Science and Technology,Tianjin 300457,China
-
Received:2021-01-18Online:2021-10-10Published:2021-10-11 -
Contact:Xu Shijie E-mail:wangyanfei@tust.edu.cn;xushijie@tust.edu.cn
CLC Number:
Cite this article
Wang Yanfei,Jiao Jian,Jiang Shuwan,Xu Shijie. Study on nucleation kinetics of sodium sulfate-water system based on inverse solubility[J]. Inorganic Chemicals Industry, 2021, 53(10): 41-46.
share this article
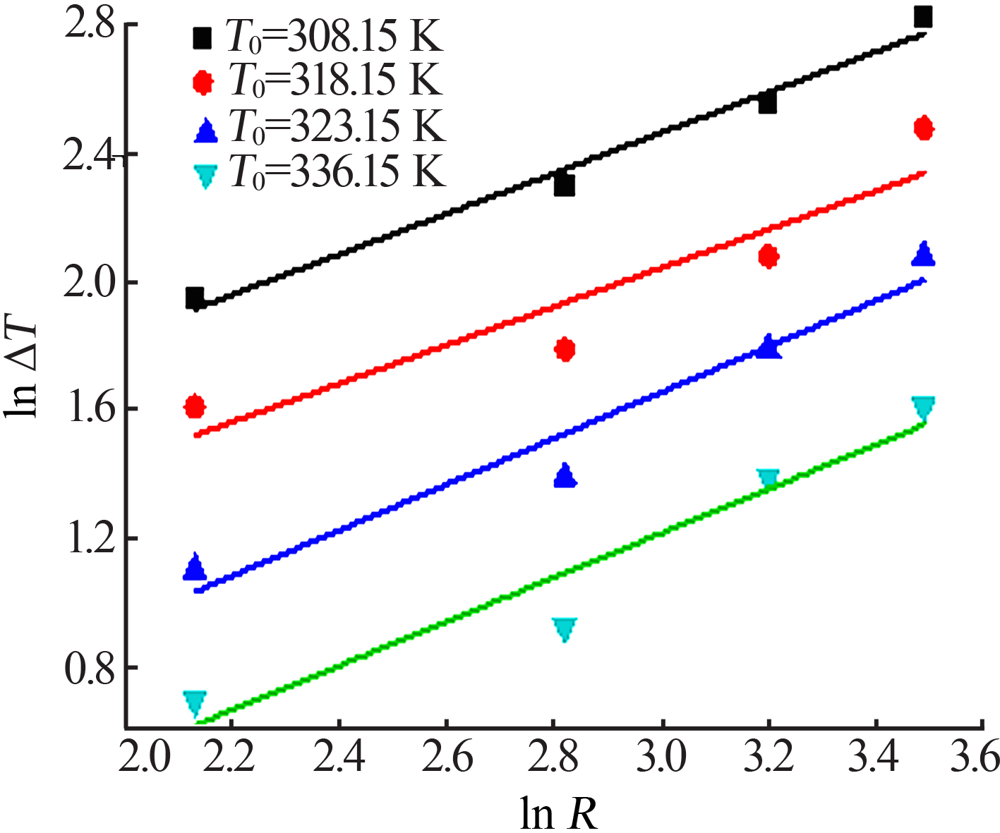
Table 1
Metastable zone data of sodium sulfate at different saturation temperature and different heating rates"
| R/(K·h-1) | ΔT/K | R/(K·h-1) | ΔT/K | R/(K·h-1) | ΔT/K | R/(K·h-1) | ΔT/K | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0=308.15 K | T0=318.15 K | T0=323.15 K | T0=336.15 K | ||||||||
| 8.4 | 7.0 | 5.8 | 5.0 | 2.5 | 3.0 | 12.0 | 2.0 | ||||
| 16.7 | 10.0 | 9.7 | 6.0 | 7.7 | 4.0 | 16.7 | 2.5 | ||||
| 24.6 | 13.0 | 21.8 | 8.0 | 16.4 | 6.0 | 26.7 | 4.0 | ||||
| 32.9 | 17.0 | 32.7 | 12.0 | 24.0 | 8.0 | 30.0 | 5.0 | ||||
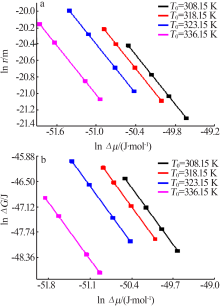
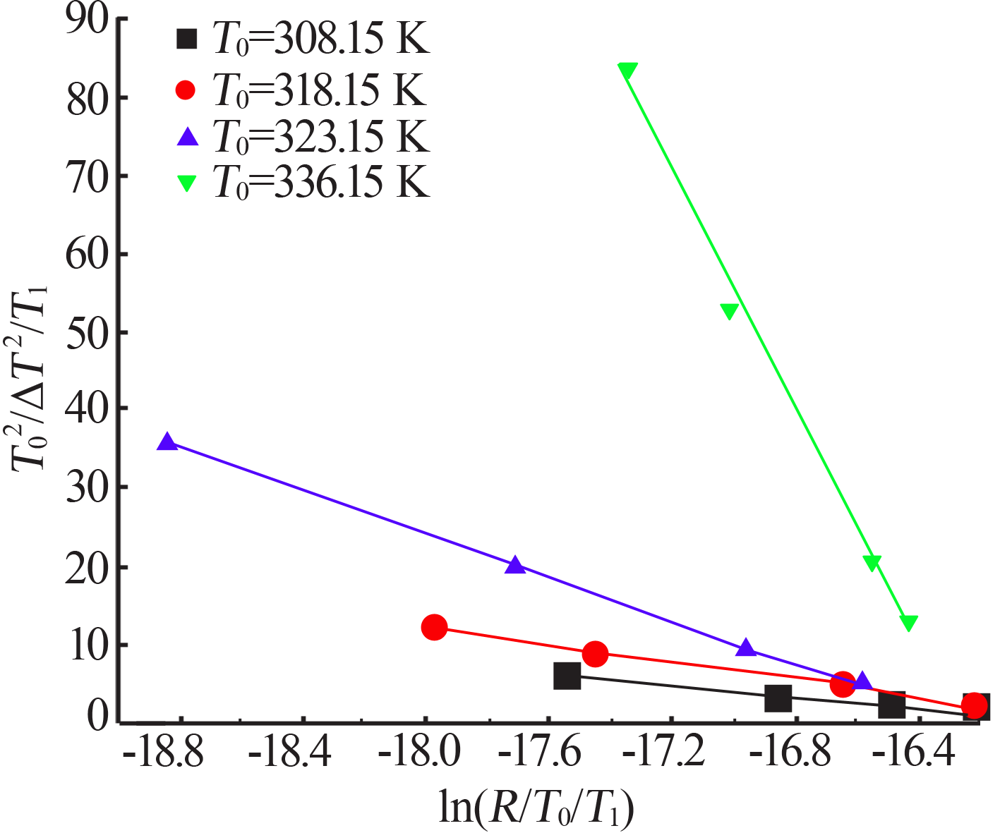
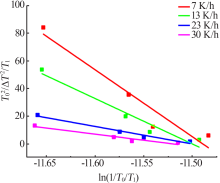
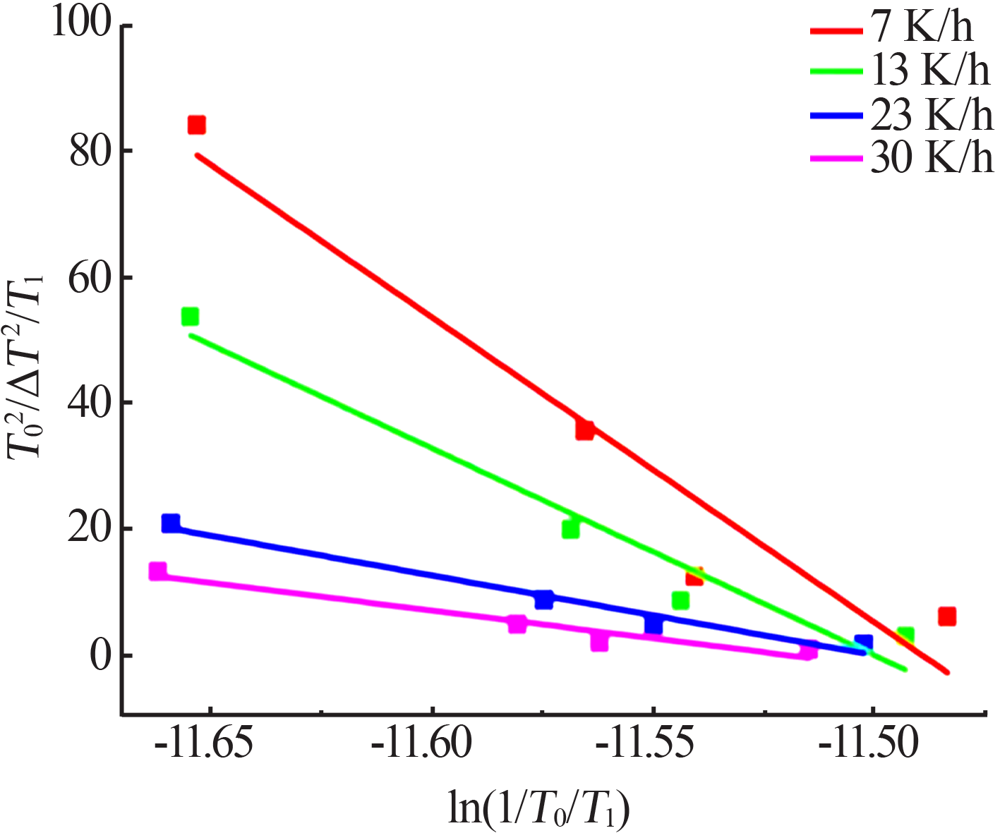
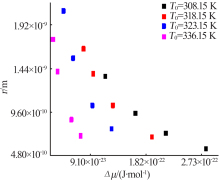
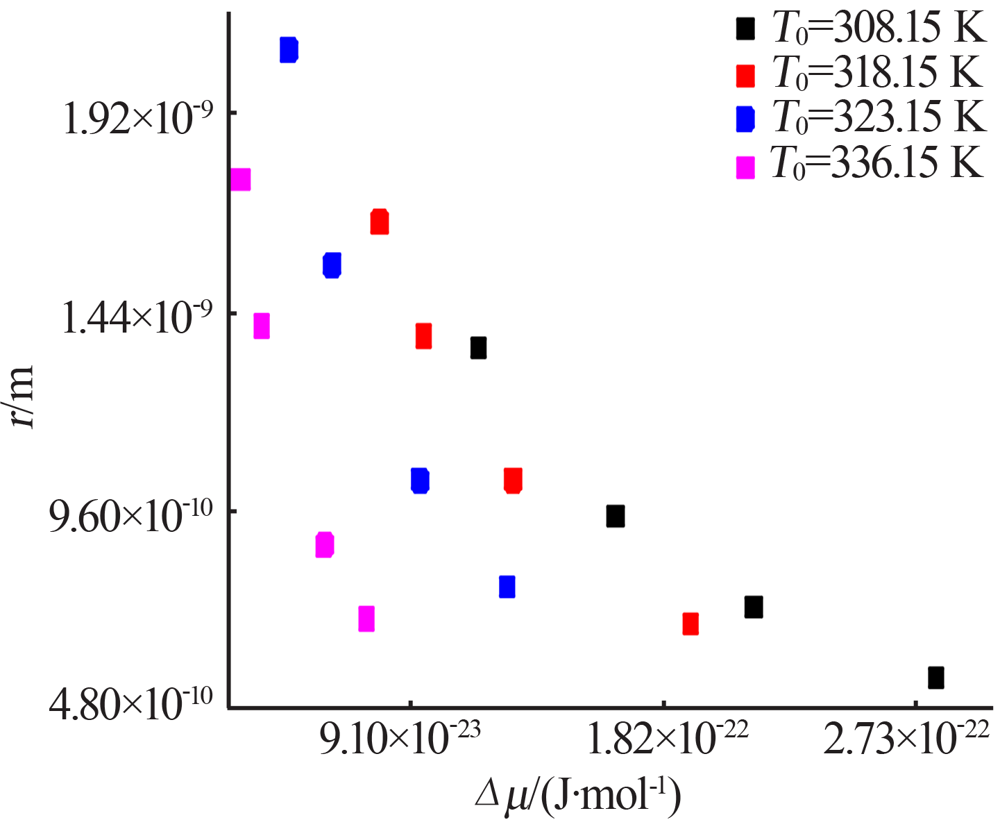
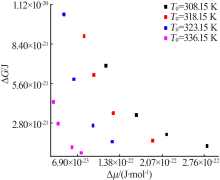
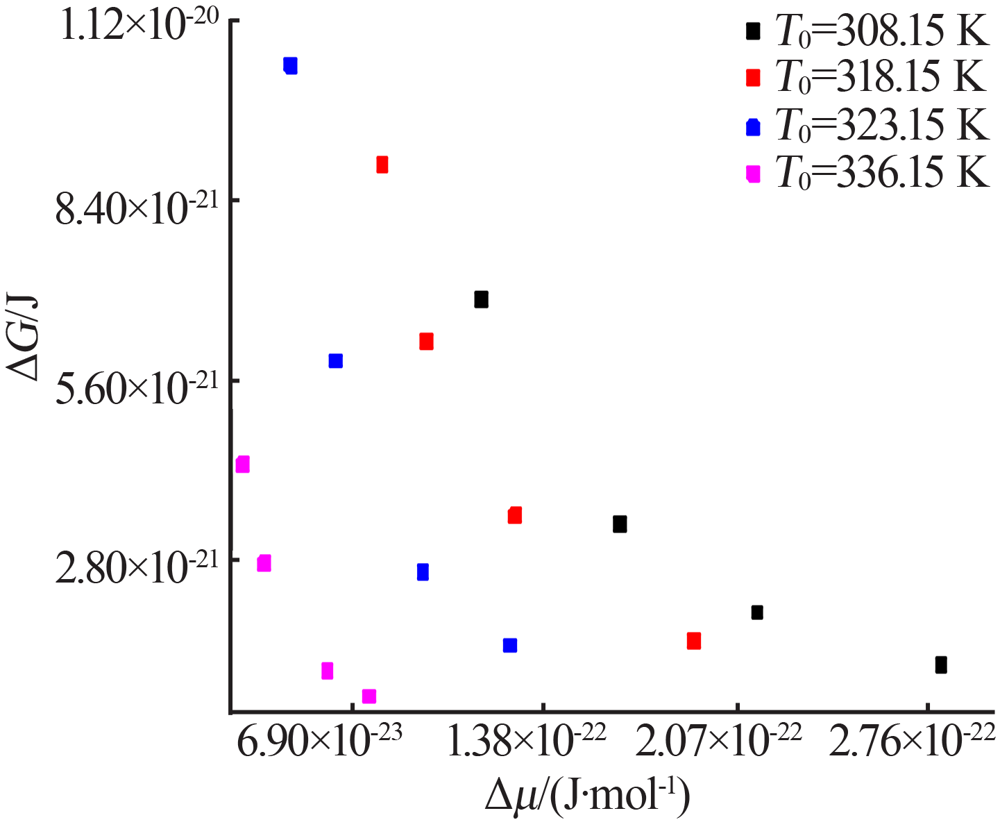
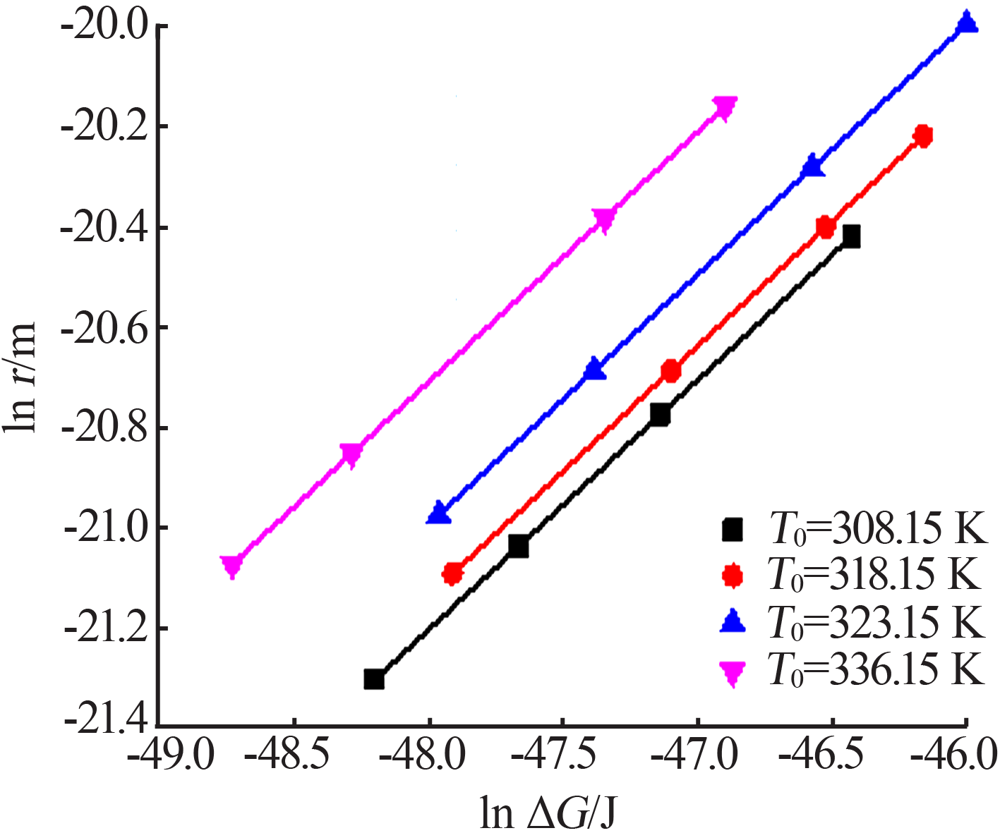
Fig.9
The relationship between the critical size and the chemical potential at different saturation temperature and corresponding heating rate(a);The relationship between the critical Gibbs free energy and the chemical potential at different saturation temperature and corresponding heating rate(b)"
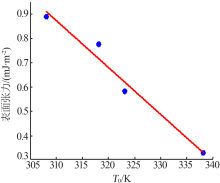
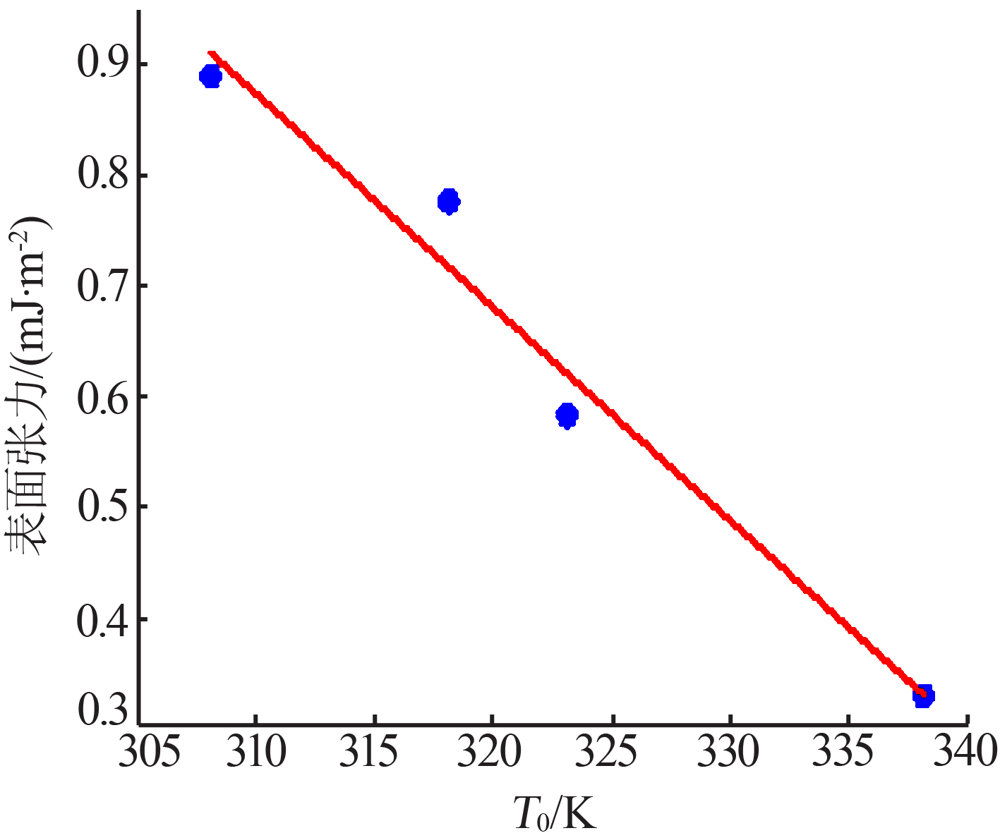
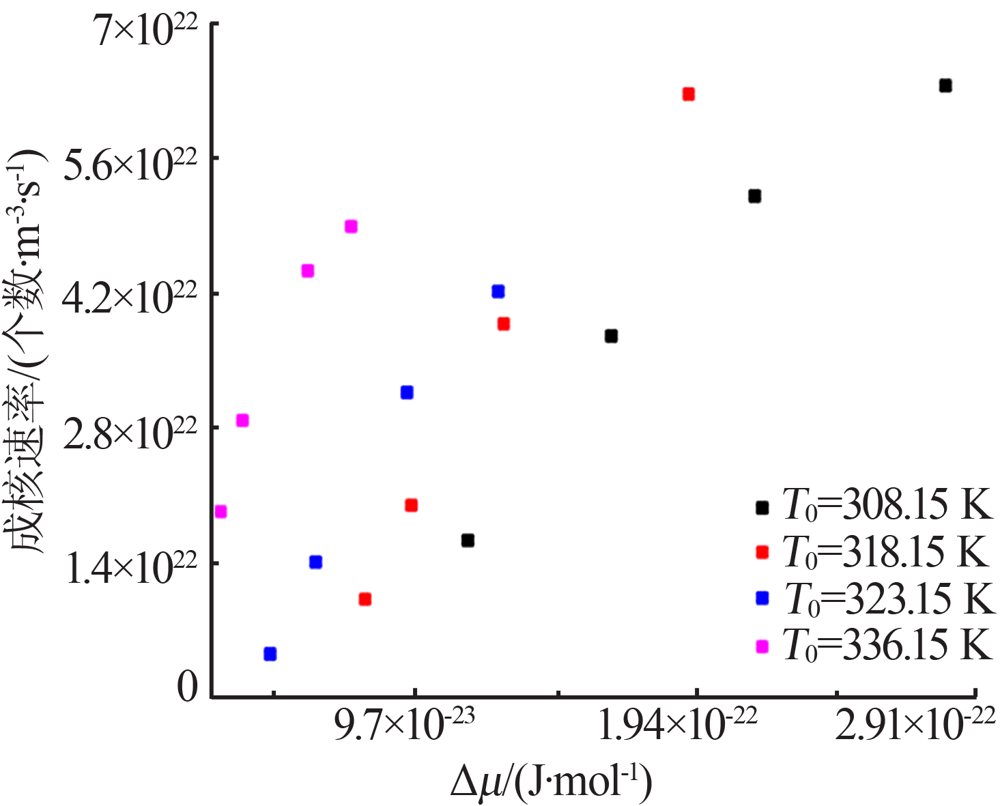
Table 5
Values of chemical potential at different saturation temperature and different degree of superheat"
| ΔT/ K | Δμ/10-22 (J·mol-1) | ΔT/ K | Δμ/10-22 (J·mol-1) | ΔT/ K | Δμ/10-22 (J·mol-1) | ΔT/ K | Δμ/10-22 (J·mol-1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0=308.15K | T0=318.15K | T0=323.15K | T0=336.15K | ||||||||
| 7 | 1.55 | 5 | 0.80 | 3 | 0.47 | 2.0 | 0.30 | ||||
| 10 | 1.65 | 6 | 0.96 | 4 | 0.63 | 2.5 | 0.38 | ||||
| 13 | 2.14 | 8 | 1.28 | 6 | 0.94 | 4.0 | 0.60 | ||||
| 17 | 2.80 | 12 | 1.92 | 8 | 1.26 | 5.0 | 0.75 | ||||
| [1] | 陶道敏. 美国亚硫酸钠生产现状[J]. 无机盐工业, 2005, 37(6):61. |
| [2] | 周永学, 王倩, 张筱军. 芒硝的临床运用与药理研究[J]. 陕西中医学院学报, 2007, 30(1):54-55. |
| [3] |
Brabants G, Hubin M, Reichel E K, et al. Revisiting silicalite-1 nu-cleation in clear solution by electrochemical impedance spectrosco-py[J]. Langmuir:The ACS Journal of Surfaces and Colloids, 2017, 33(10):2581-2589.
doi: 10.1021/acs.langmuir.6b04135 |
| [4] |
Davey R J. Can the study of self-assembly in solution lead to a good model for the nucleation pathway:The case of tolfenamic acid[J]. Chemical Science, 2015, 6(6):3515-3524.
doi: 10.1039/C5SC00522A |
| [5] |
Chiarella R A, Gillon A L, Burton R C, et al. The nucleation of ino-sine:the impact of solution chemistry on the appearance of polymo-rphic and hydrated crystal forms[J]. Faraday Discussions, 2007, 136:179-193.
pmid: 17955810 |
| [6] | Xiong Lixuan, Ling Zhou, Zhang Xia, et al. Determination of metasta-ble zone widths and nucleation behavior of aspirin in acetic acid and acetic anhydride binary solvent mixture[J]. Journal of Molecu-lar Liquids, 2018, 269:805-815. |
| [7] | Somchai M, Flood A E. Validation of models predicting nucleation rates from induction times and metastable zone widths[J]. Chemical Engineering & Technology, 2018, 41(10):2066-2076. |
| [8] |
Quan Y, Yang Y, Xu S, et al. Insight into the role of piperazine in the thermodynamics and nucleation kinetics of the triethylenediamine-methyl tertiary butyl ether system[J]. Crystengcomm, 2019, 21:948-956.
doi: 10.1039/C8CE01179F |
| [9] |
Ma S, Li C, Gao J, et al. Artificial neural network prediction of me-tastable zone widths in reactive crystallization of lithium carbona-te[J]. Industrial & Engineering Chemistry Research, 2020.Doi: 10.1021/acs.iecr.9b06074.
doi: 10.1021/acs.iecr.9b06074 |
| [10] |
Xu S J, Bu Y Q, Jiang S W, et al. Insights into the role of solvents in nucleation kinetics of glutaric acid from metastable zone widths[J]. Industrial & Engineering Chemistry Research, 2021.Doi: 10.1021/acs.iecr.0c04368.
doi: 10.1021/acs.iecr.0c04368 |
| [11] |
Yang H, Florence A J. Relating induction time and metastable zone width[J]. CrystEngComm, 2017, 19(28):3966-3978.
doi: 10.1039/C7CE00770A |
| [12] |
Noor S Z M, Camacho D M, Ma C Y, et al. The effect of crystallization conditions on the metastable zone width and nucleation kinetics of para-aminobenzoic acid in ethanol[J]. Chemical Engineering & Technology, 2020, 43(6).Doi: 10.1002/ceat.201900679.
doi: 10.1002/ceat.201900679 |
| [13] | Sangwal K. Recent developments in understanding of the metasta-ble zone width of different solutesolvent systems[J]. Journal of Cry-stal Growth, 2011, 318(1):103-109. |
| [14] |
Kashchiev D, Borissova A, Hammond R B, et al. Effect of cooling rate on the critical undercooling for crystallization[J]. Journal of Crystal Growth, 2010, 312(5):698-704.
doi: 10.1016/j.jcrysgro.2009.12.031 |
| [15] |
Yang J, Xu S, Wang J, et al. Nucleation behavior of ethyl vanillin:Balance between chemical potential difference and saturation temperature[J]. Journal of Molecular Liquids, 2020, 303.Doi: 10.1016/j.molliq.2020.112609.
doi: 10.1016/j.molliq.2020.112609 |
| [1] | WANG You, LIAO Lianzhen, CHEN Zheng, GAO Youjun. Effect of surfactants on electrocrystallization of Ni(OH)2 [J]. Inorganic Chemicals Industry, 2025, 57(3): 58-63. |
| [2] | ZHU Jicheng, YANG Qixin, LIANG Haoquan, WANG Zengkun, OUYANG Fugui, DI Jing, GAI Xikun. Effect of confined catalyst Ni@S2 on performance of methane dry reforming reaction [J]. Inorganic Chemicals Industry, 2025, 57(2): 138-146. |
| [3] | ZOU Yang, LU Zhiyan, HU Zhilin, SUN Ze. Study on metastable zone width and primary nucleation kinetics for cooling crystallization of KNO3 [J]. Inorganic Chemicals Industry, 2024, 56(9): 67-74. |
| [4] | LI Shuai, LI Tianxiang, ZHU Jing, LIU Songlin. Study on purification process of sodium fluoride [J]. Inorganic Chemicals Industry, 2024, 56(9): 90-97. |
| [5] | WANG Jianjie, SHU Xiaolong, XIAO Xia, WANG Peng, FAN Xiaoqiang, KONG Lian, XIE Zean, ZHAO Zhen. Study on synthesis of hierarchical flower⁃like ZSM-5 zeolite and its catalytic performance for n-octane cracking [J]. Inorganic Chemicals Industry, 2024, 56(8): 139-146. |
| [6] | SU Hang, SONG Jitian, HUANG Zhiqiang, DONG Qing, ZHANG Yaxiong. Study on crystallization kinetics of manganese sulfate monohydrate in H2SO4-H2O binary system [J]. Inorganic Chemicals Industry, 2024, 56(8): 40-46. |
| [7] | GUO Kaihua, FAN Yuxin, YANG Jing, ZHAO Wenli, JIA Yuanyuan, WANG Yanfei. Analysis of effect of carnallite raw ore grade on its cold decomposition and crystallization of potassium chloride [J]. Inorganic Chemicals Industry, 2024, 56(8): 9-18. |
| [8] | CHENG Chunchun, LI Yulong, ZHANG Zhiqiang, LIU Xuejing. Study on dissolution crystallization for extraction of potassium and separation of magnesium and lithium from salt lake brine [J]. Inorganic Chemicals Industry, 2024, 56(6): 34-39. |
| [9] | LIU Xiaowen, LI Jun, ZHOU Zhaoan, MAO Anzhang, ZHOU Aiqing. Study on response surface methodology optimization of PAC for deep purification of fluorine ion in high concentration sodium sulfate solution [J]. Inorganic Chemicals Industry, 2024, 56(6): 67-72. |
| [10] | HU Cheng, LIU Meng, XIANG Weiheng, DUAN Pengxuan, LI Shunkai, MING Yang, WANG Neng, LU Guanju. Effect of NaCl solution concentration on transcrystallization behavior of α-hemihydrate gypsum from phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(6): 87-93. |
| [11] | LI Yuxing, ZHANG Jincai, CHENG Fangqin. Research progress of preparation and growth mechanism of various crystalline nano-calcium carbonate [J]. Inorganic Chemicals Industry, 2024, 56(5): 1-10. |
| [12] | LI Chunli, ZHANG Huanhuan, CHENG Zhuo, TANG Xiuhua, ZHANG Fengzhen, YE Yuling. Anti-solvent crystallization process of NH4VO3 in NaVO3-NH4Cl-H2O solution system [J]. Inorganic Chemicals Industry, 2024, 56(5): 39-44. |
| [13] | LUO Ya, ZHOU Rong, LÜ Li, YANG Jie, TANG Shengwei, ZHANG Tao. Study on crystallization and filtration properties of calcium sulfate for treating industrial waste sulfuric acid with calcium carbonate [J]. Inorganic Chemicals Industry, 2024, 56(12): 127-133. |
| [14] | LI Hongyuan, ZHANG Jianhua. Study on removal process of total organic carbon from industrial waste salts by pyrolysis [J]. Inorganic Chemicals Industry, 2024, 56(10): 95-102. |
| [15] | WEI Tianshun, JI Lijun, SHENG Yong, CHEN Kui, WU Yanyang, WU Bin. Study on fractional crystallization process of ammonium sulfate and sodium sulfate in high salt wastewater [J]. Inorganic Chemicals Industry, 2024, 56(1): 102-106. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||