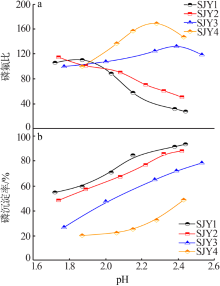
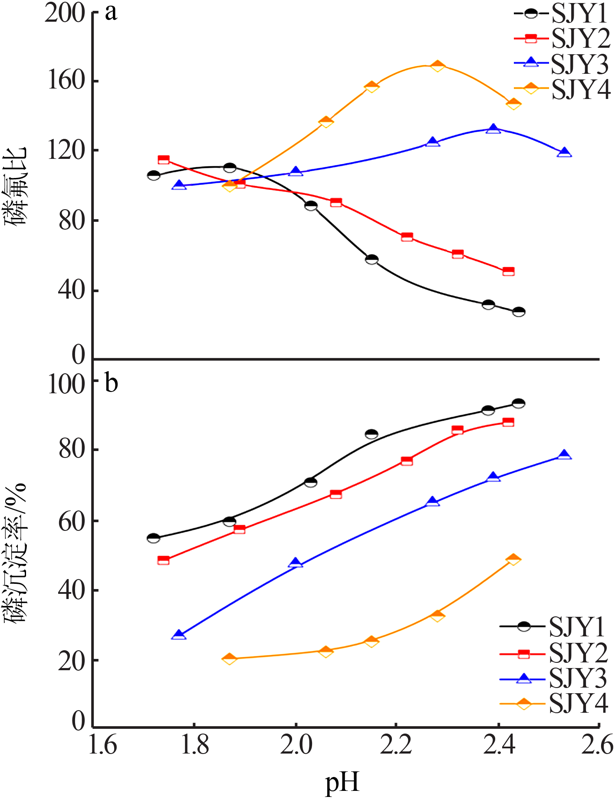
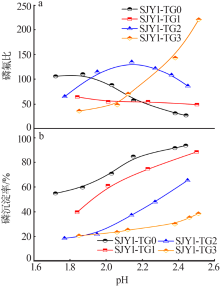
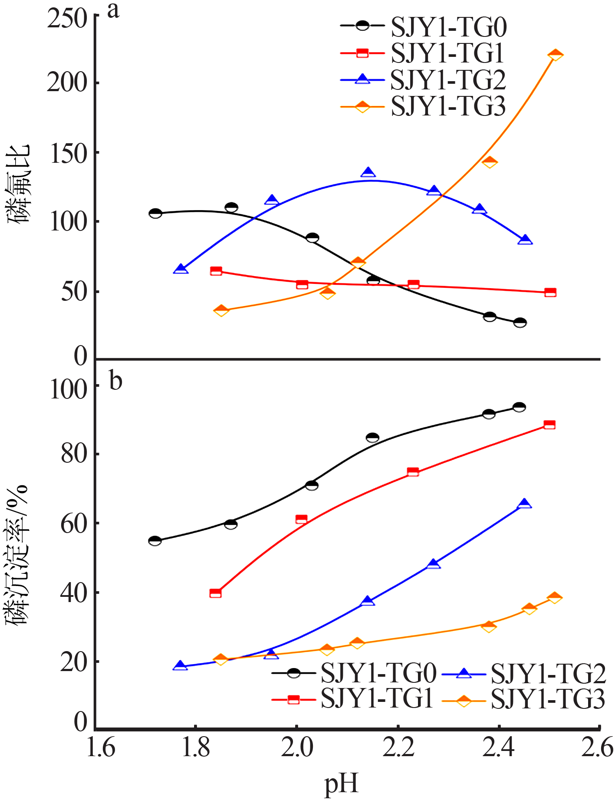
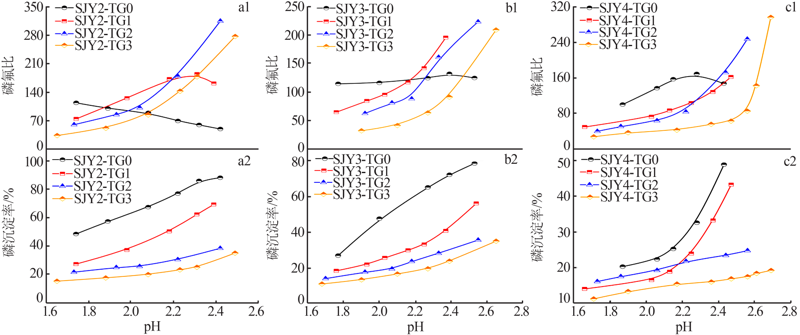
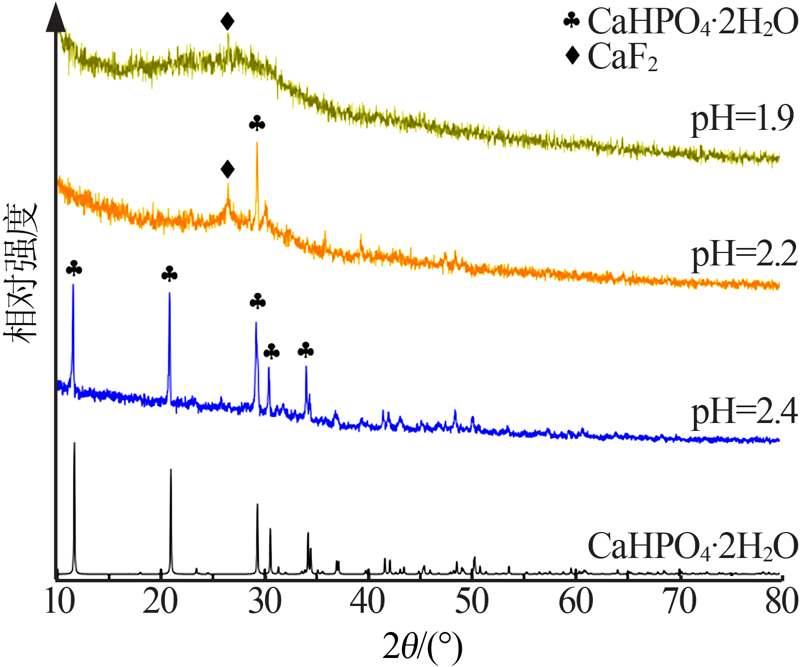
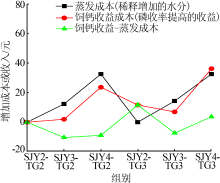
| [1] |
王喜恒, 孙文哲. 湿法磷酸过程氟回收技术研究进展[J]. 无机盐工业, 2020, 52(8):25-29.
|
| [2] |
陈雪萍. 湿法磷酸脱氟净化研究[D]. 昆明:昆明理工大学, 2007.
|
| [3] |
陶长元, 王秀秀, 刘作华, 等. 湿法磷酸浸出强化及有机质去除研究[J]. 化工学报, 2020, 71(10):4792-4799.
|
| [4] |
段利中. 湿法磷酸制饲料级磷酸氢钙清洁除氟工艺的研究[D]. 武汉:武汉科技大学, 2011.
|
| [5] |
任孟伟. 湿法磷酸反应过程脱氟技术研究[D]. 郑州:郑州大学, 2018.
|
| [6] |
王斌, 张宗凡, 罗康碧, 等. 盐酸法湿法磷酸工艺研究现状[J]. 化学工程师, 2014(8):46-49.
|
| [7] |
顾春光. 硝酸分解磷矿综合利用设想[J]. 硫磷设计与粉体工程, 2013(1):18-23,49.
|
| [8] |
张鑫, 钟本和, 孙玉柱. 生产饲料级磷酸氢钙的新工艺[J]. 现代化工, 2015, 35(1):158-159.
|
| [9] |
周江润, 许磊, 陶辉, 等. 料浆直接法生产饲料级磷酸氢钙工艺技术简析[J]. 磷肥与复肥, 2019, 34(12):18-19,22.
|
| [10] |
任孟伟, 汤建伟, 芦雷鸣, 等. 湿法磷酸工艺脱氟研究[J]. 无机盐工业, 2018, 50(10):14-16,44.
|
| [11] |
黄忠, 余双强, 饶济, 等. 低氟磷酸浓缩脱氟工艺研究[J]. 化工矿物与加工, 2020, 49(6):50-53.
|
| [12] |
田文航, 郝易潇, 赵军, 等. 湿法磷酸净化中的深度脱氟技术[J]. 磷肥与复肥, 2016, 31(3):31-32,48.
|
| [13] |
何宾宾, 周琼波, 张晖, 等. 湿法磷酸汽提法脱氟技术研究[J]. 无机盐工业, 2016, 48(9):49-50.
|
| [14] |
张海燕, 明大增, 吉晓玲, 等. 浅析湿法磷酸脱氟反应原理[J]. 无机盐工业, 2015, 47(1):9-12.
|
| [15] |
张海燕. 湿法磷酸深度脱氟精制食品级磷酸工艺研究[D]. 昆明:昆明理工大学, 2015.
|
| [16] |
阳杨, 盛勇, 周佩, 等. 湿法磷酸浓缩后的氟分配及脱氟工艺研究[J]. 磷肥与复肥, 2015, 30(9):31-33,37.
|
| [17] |
王保华. 湿法磷酸净化脱氟的实验研究[J]. 化工矿物与加工, 2014, 43(7):14-16.
|
| [18] |
赵明. 盐酸法饲料磷酸氢钙脱氟机理研究[D]. 成都:四川大学, 2004.
|
| [19] |
唐明亮, 何明凯. 湿法磷酸生产过程氟平衡及提高氟收率措施研究[J]. 硫磷设计与粉体工程, 2020(3):1-3,5.
|
 ),Xu Dehua(
),Xu Dehua( ),Li Chaorong,Yang Xiushan,Wang Xinlong,Zhang Zhiye(
),Li Chaorong,Yang Xiushan,Wang Xinlong,Zhang Zhiye( )
)