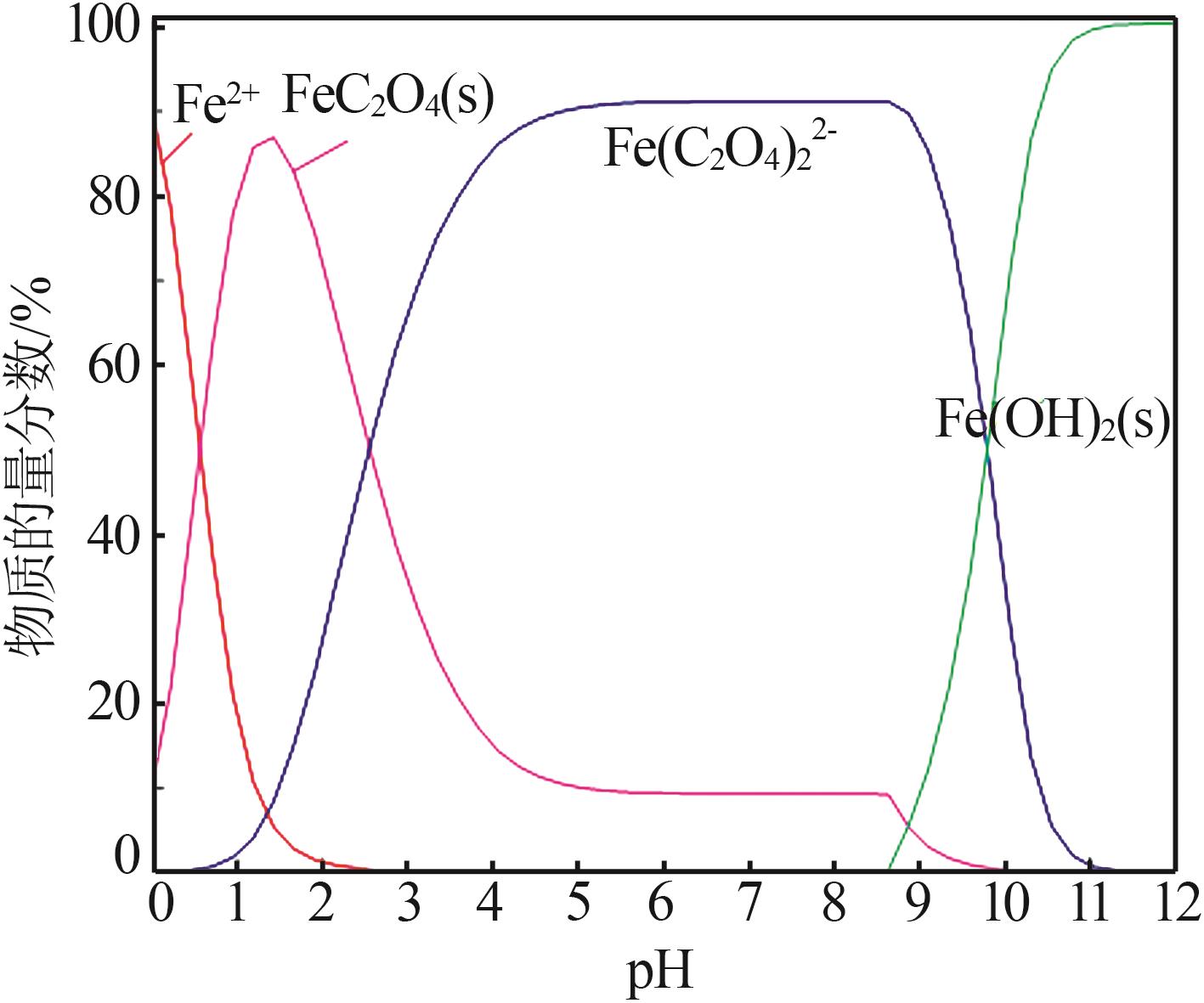
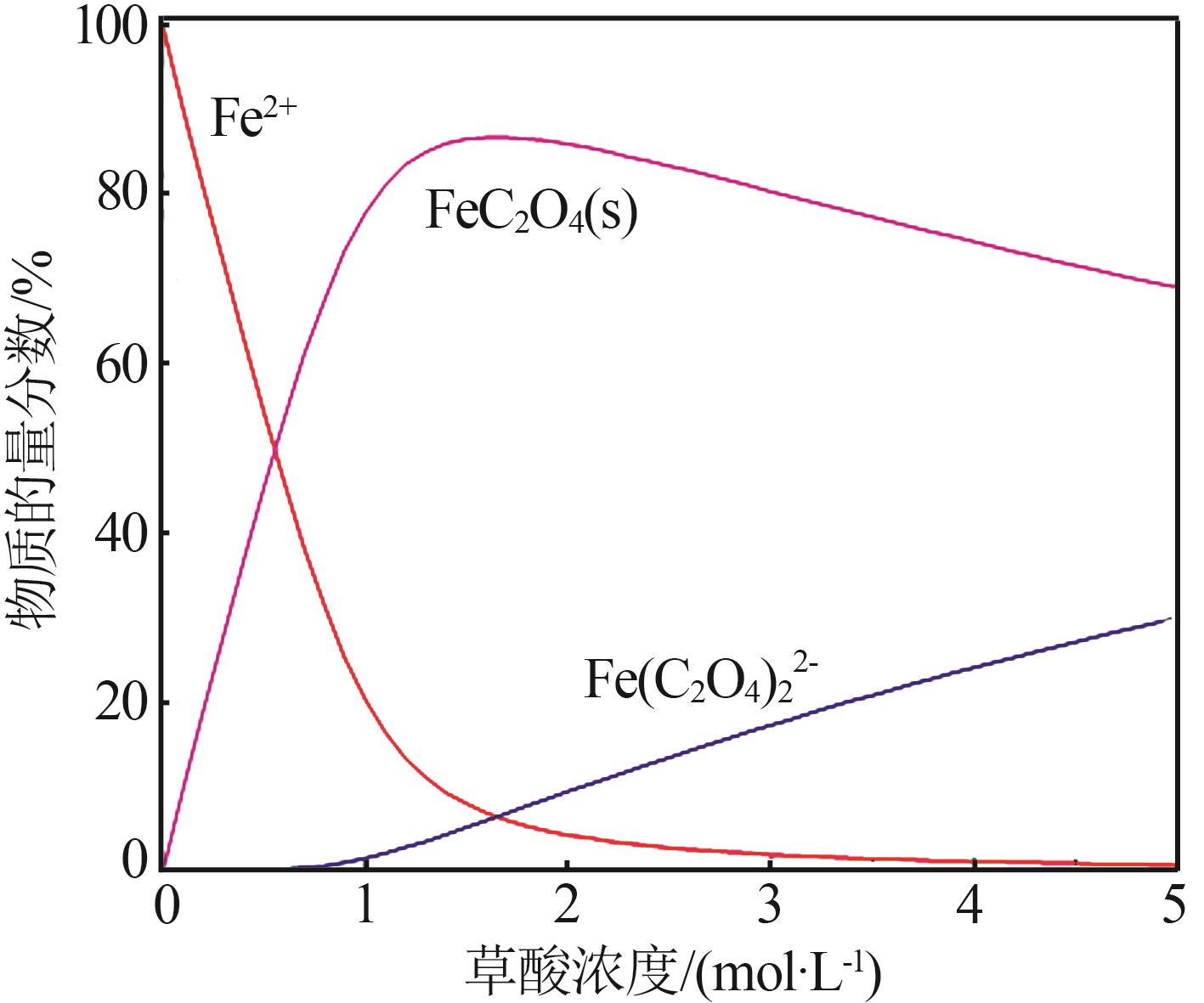
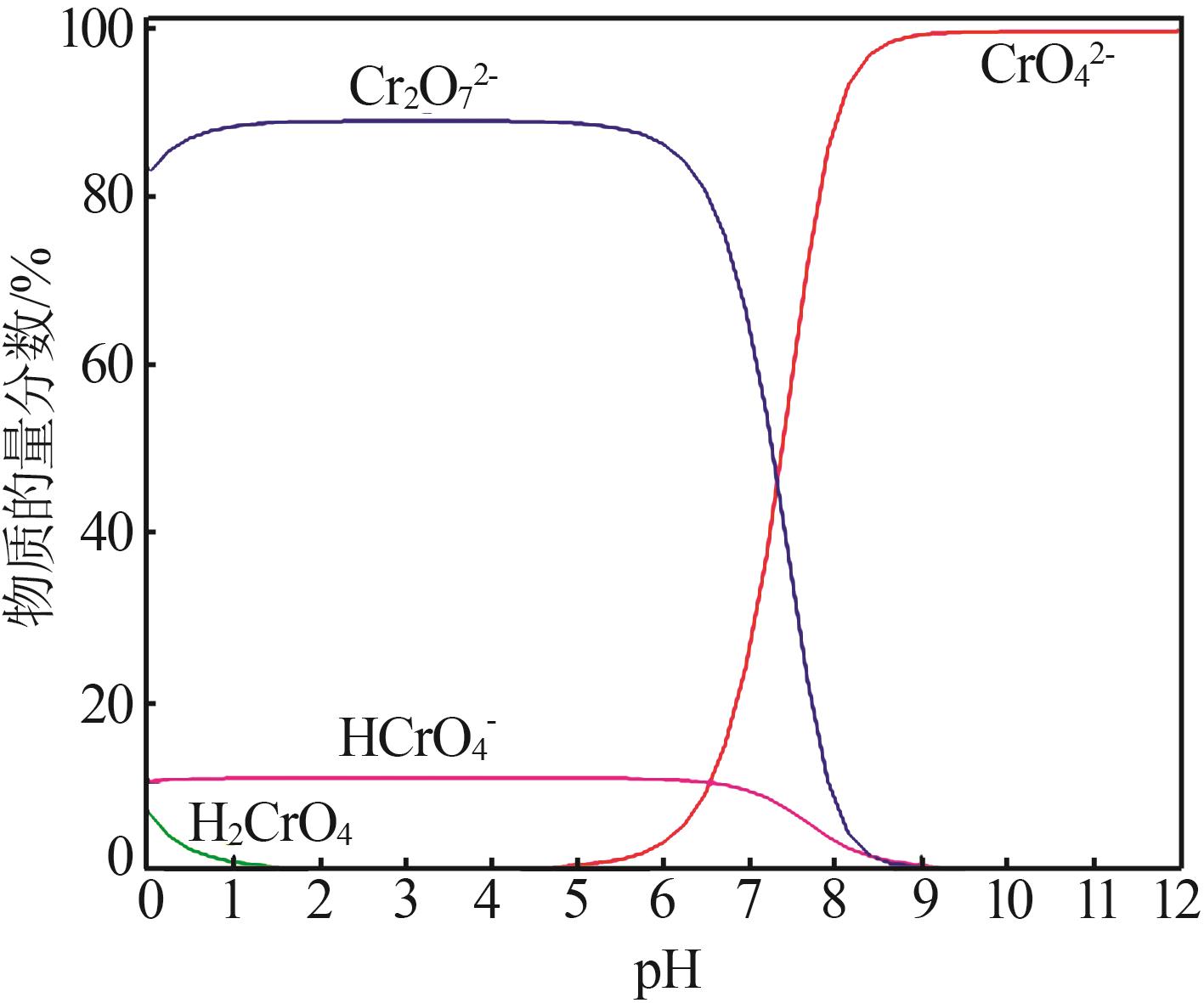
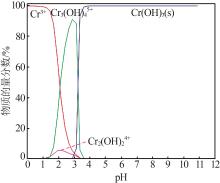
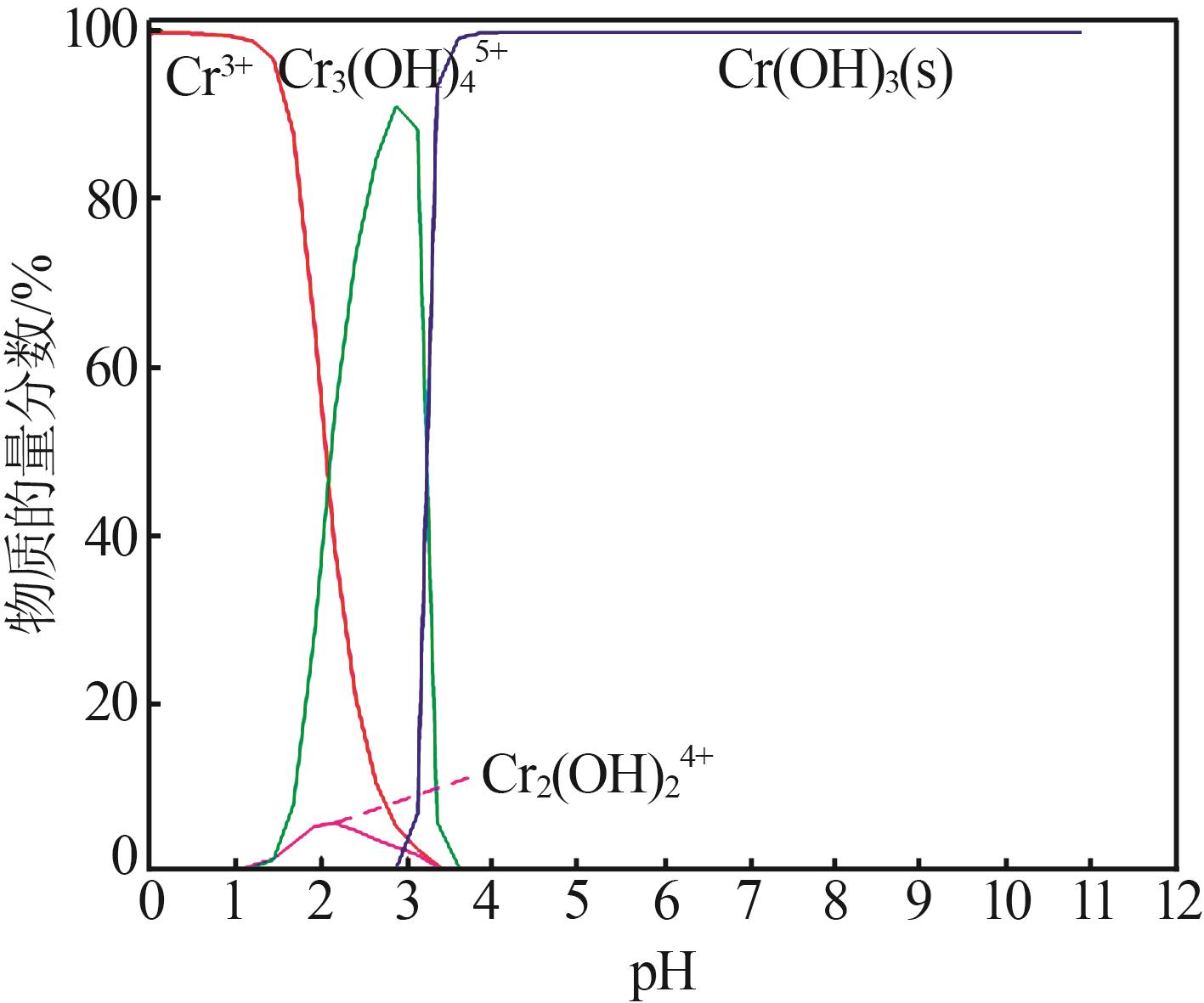
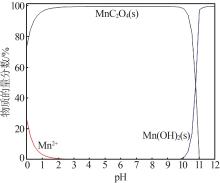
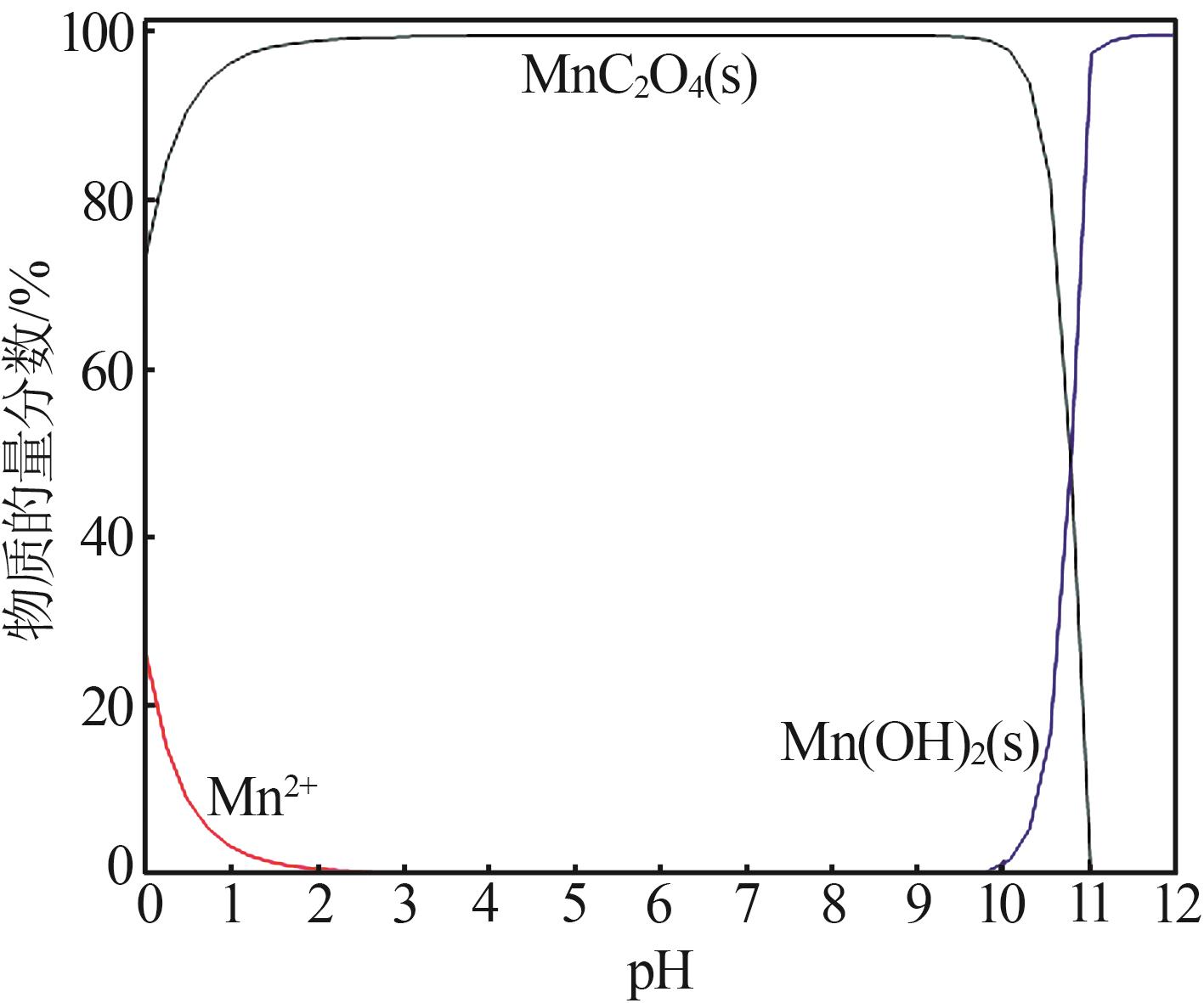
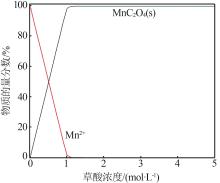
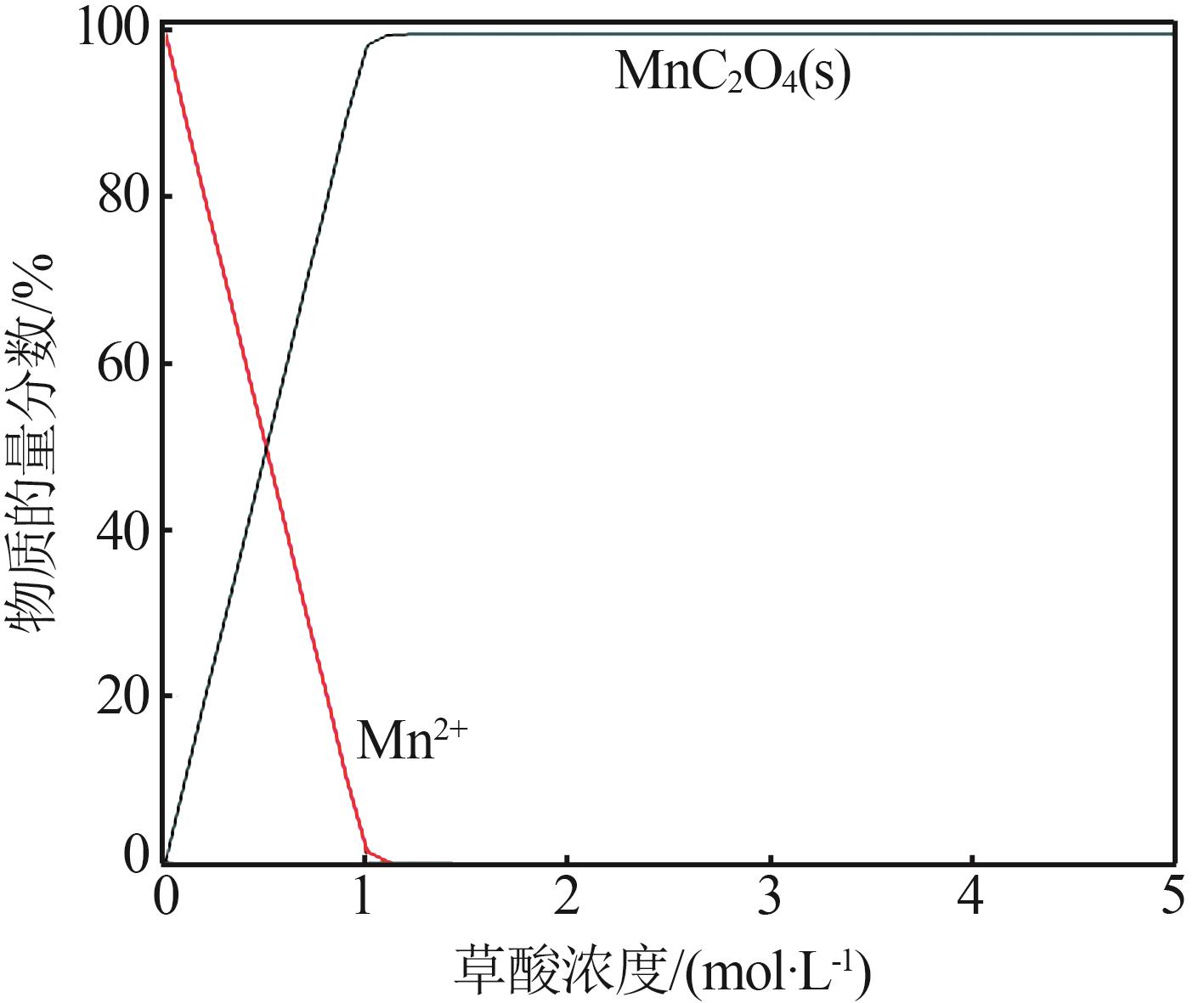
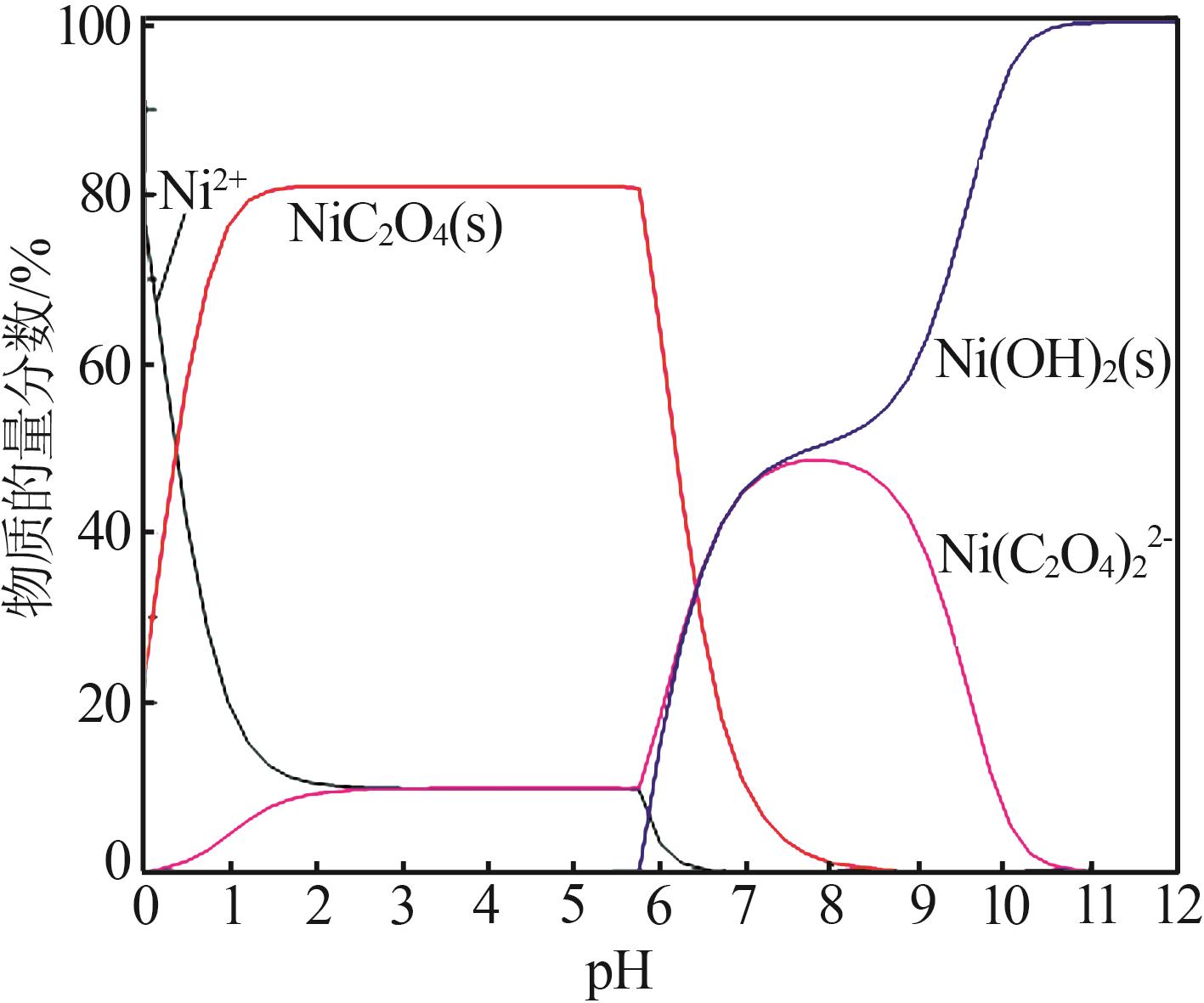
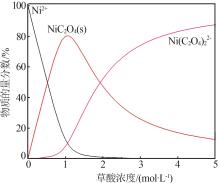
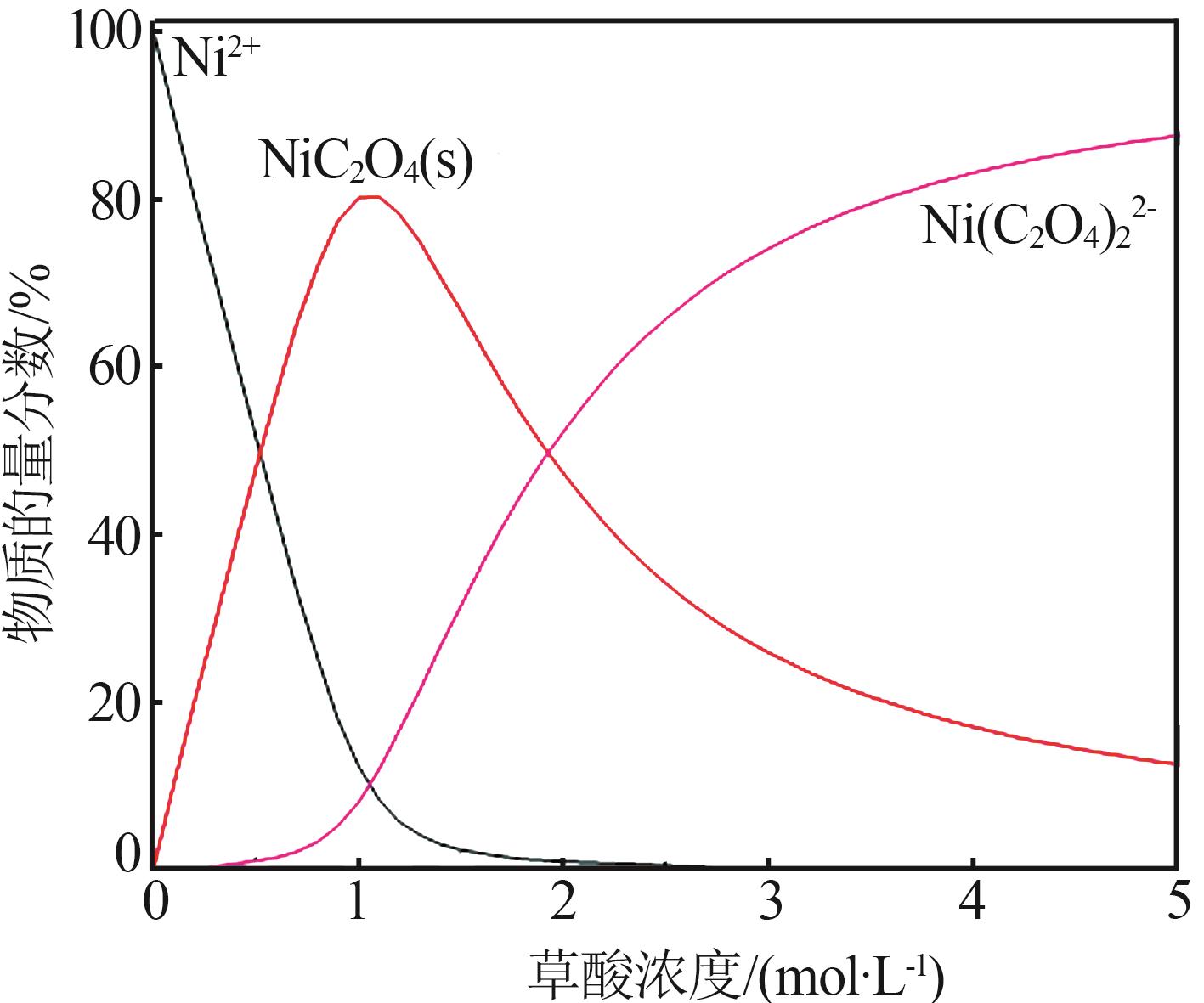
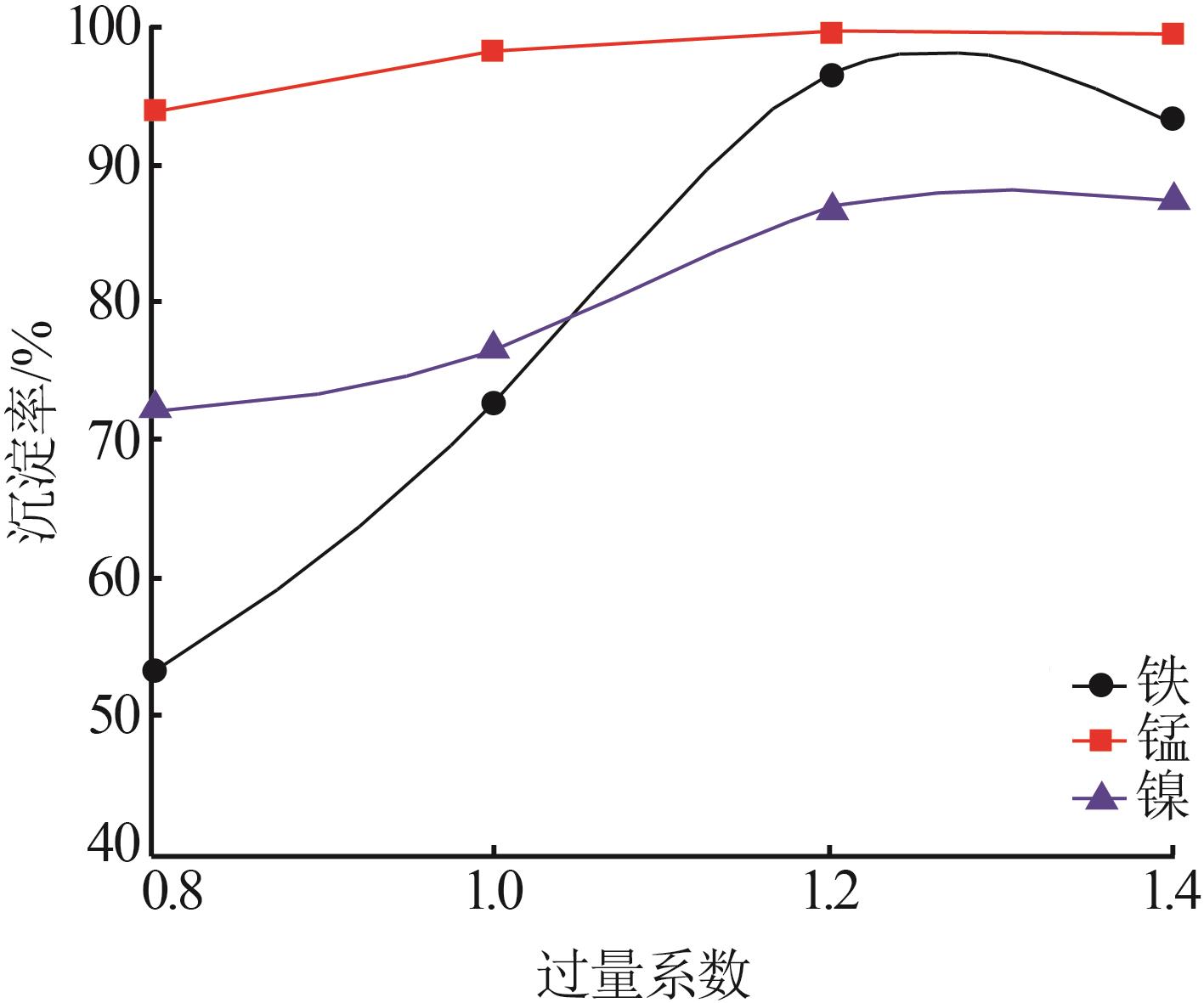
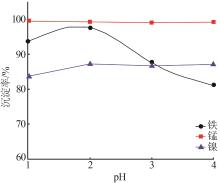
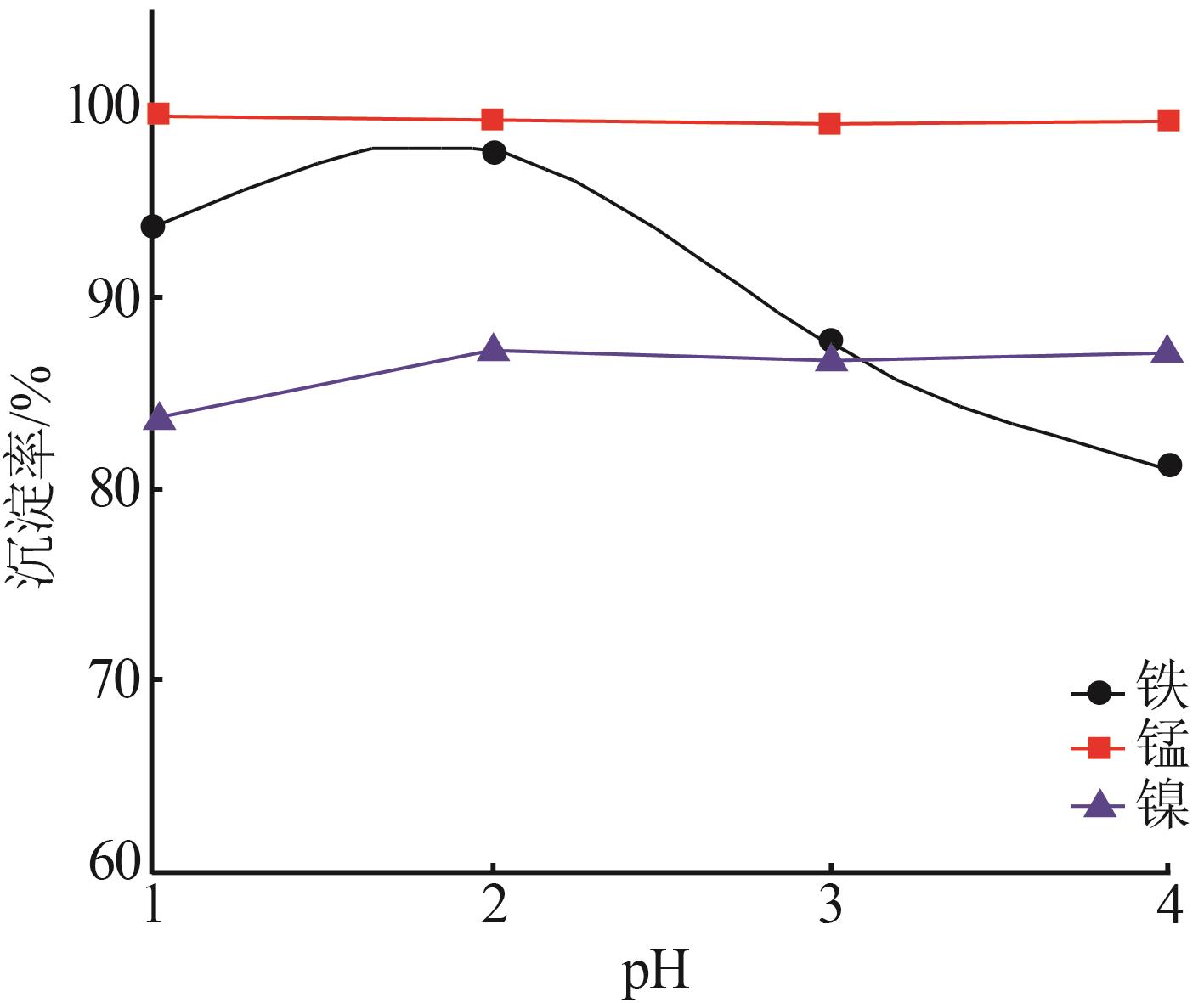
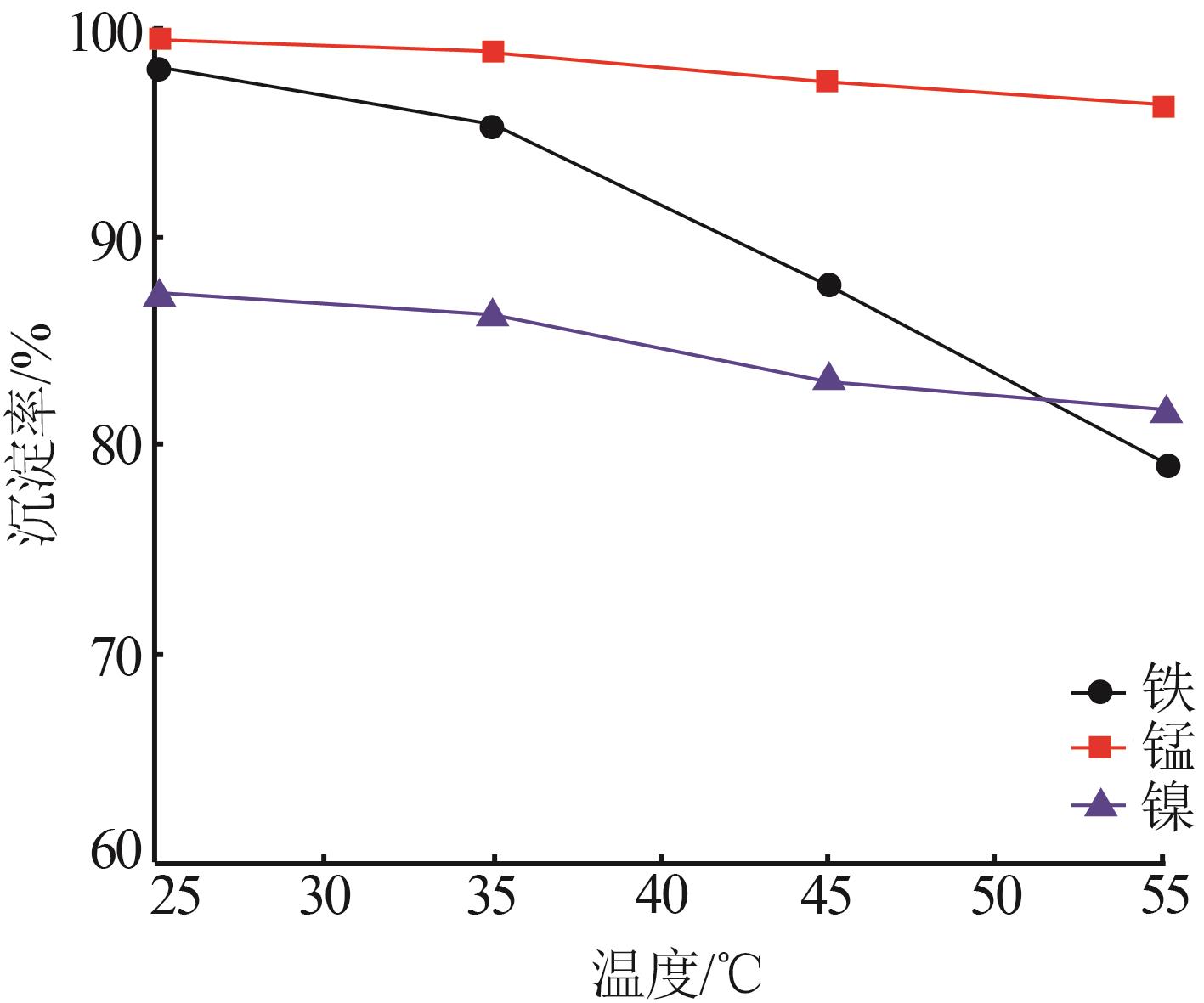
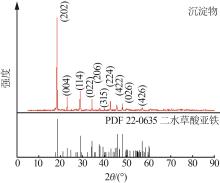
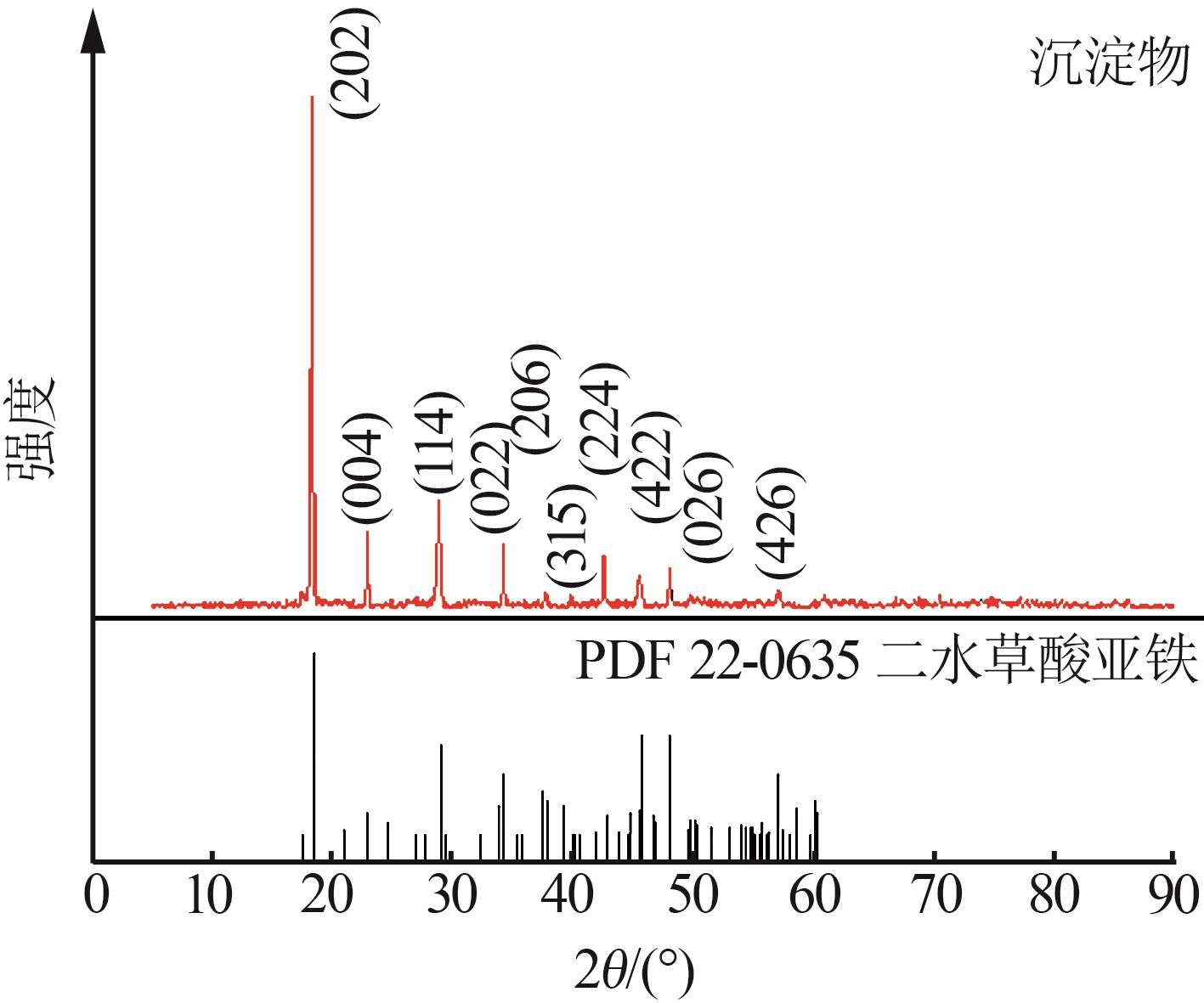
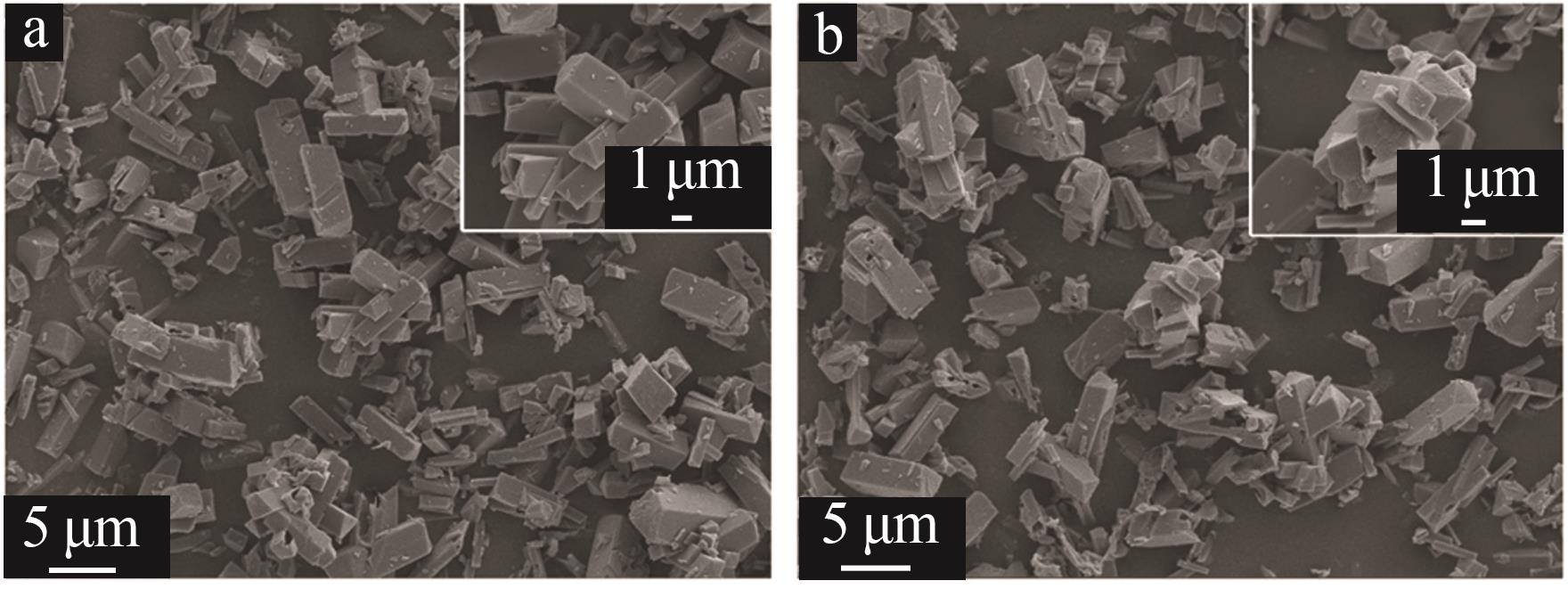
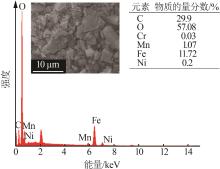
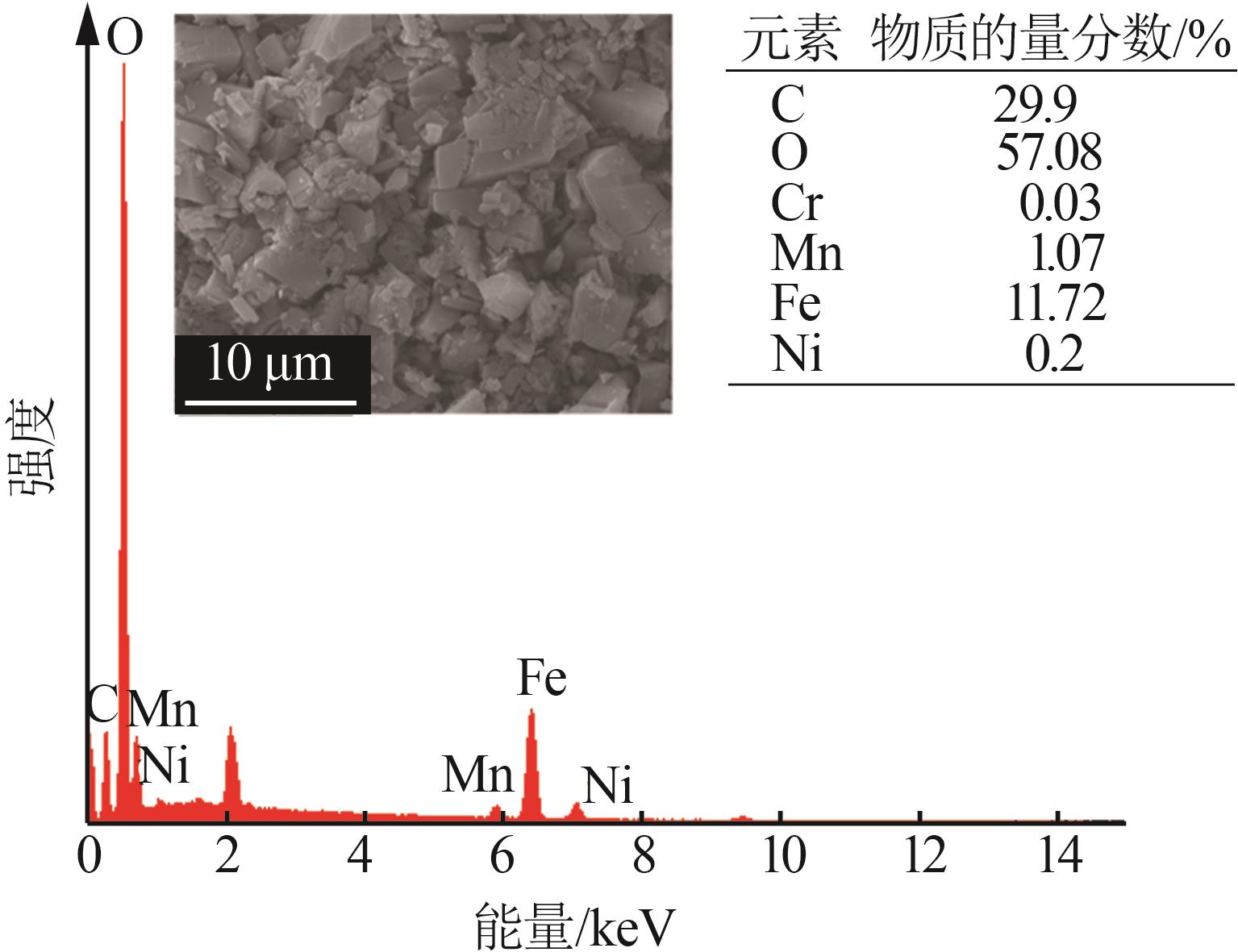
| 1 |
王海人,王宜民,屈钧娥,等.不锈钢着色方法及表征技术[J].材料保护,2012,45(12):38-41,45,1.
|
|
WANG Hairen, WANG Yimin, QU June,et al.Research progresses in coloring methods of stainless steel and characterization techniques of coloring films[J].Materials Protection,2012,45(12):38-41,45,1.
|
| 2 |
王玥,满瑞林,赵鹏飞,等.电化学氧化法再生不锈钢着色老化液的研究[J].表面技术,2012,41(5):114-118.
|
|
WANG Yue, MAN Ruilin, ZHAO Pengfei,et al.Study on regeneration of ageing liquid for stainless steel coloring by electrochemical oxidation[J].Surface Technology,2012,41(5):114-118.
|
| 3 |
LI Wenwei, QU Jun'e, CAO Zhiyong,et al.Effects of NiSO4 concentration on the coloring performance and corrosion resistance of the colored film on 304 stainless steel[J].Coatings,2020,10(6).Doi:10.3390/coatings10060598 .
doi: 10.3390/coatings10060598
|
| 4 |
王海人,李文维,屈钧娥,等.Cr3+和Fe3+对不锈钢着色液老化进程的协同影响[J].表面技术,2011,40(2):29-32.
|
|
WANG Hairen, LI Wenwei, QU June,et al.Synergy effect of Cr3+ and Fe3+ on the aging process of coloring liquid for stainless st-eel[J].Surface Technology,2011,40(2):29-32.
|
| 5 |
汤芝平,薛永强,程作慧,等.Cr3+,Fe3+和Ni2+对不锈钢着色的影响[J].材料保护,2008,41(9):24-27,85.
|
|
TANG Zhiping, XUE Yongqiang, CHENG Zuohui,et al.Effect of metallic ion impurities on coloring of stainless steel[J].Materials Protection,2008,41(9):24-27,85.
|
| 6 |
张晓丽,鞠洪斌,薛永强,等.不锈钢化学着色老化液处理的新技术[J].电镀与环保,2013,33(3):39-41.
|
|
ZHANG Xiaoli, JU Hongbin, XUE Yongqiang,et al.A new technology for treating deteriorated stainless steel chemical coloring liquid[J].Electroplating & Pollution Control,2013,33(3):39-41.
|
| 7 |
秦险峰,全学军,叶鹏,等.铬铁矿酸浸过程强化及铬铁分离研究进展[J].无机盐工业,2020,52(6):13-19.
|
|
QIN Xianfeng, QUAN Xuejun, YE Peng,et al.Research progress of process intensification of acid leaching technology from chromite ore and separation of chromium-iron[J].Inorganic Chemicals Industry,2020,52(6):13-19.
|
| 8 |
邵武俊,刘春燕,贺宁,等.草酸溶液处理对SAPO-34分子筛物性和催化性能的影响[J].无机盐工业,2020,52(2):84-90.
|
|
SHAO Wujun, LIU Chunyan, HE Ning,et al.Effect of oxalic acid solution treatment on physiochemical properties and catalytic performance of SAPO-34 molecular sieve[J].Inorganic Chemicals Industry,2020,52(2):84-90.
|
| 9 |
LI Wang, YAN Xudong, NIU Zeping,et al.Selective recovery of vanadium from red mud by leaching with using oxalic acid and sodium sulfite[J].Journal of Environmental Chemical Engineering,2021,9(4).Doi:10.1016/j.jece.2021.105669 .
doi: 10.1016/j.jece.2021.105669
|
| 10 |
刘继军,林红梅,胡国荣,等.利用铬铁合金生产铬盐的新工艺研究[J].无机盐工业,2021,53(6):156-159.
|
|
LIU Jijun, LIN Hongmei, HU Guorong,et al.Study on new process of producing chromium salt with ferrochrome alloy[J].Inorganic Chemicals Industry,2021,53(6):156-159.
|
| 11 |
苏玉长,陈宏艳,胡泽星,等.不同维度草酸亚铁的合成及其组织结构[J].中南大学学报:自然科学版,2013,44(6):2237-2243.
|
|
SU Yuchang, CHEN Hongyan, HU Zexing,et al.Synthesis and microstructure of ferrous oxalate dehydrate of different dimensionalities[J].Journal of Central South University:Science and Technology,2013,44(6):2237-2243.
|
| 12 |
柯望,贾艳梅,余学峰,等.多方法制备草酸亚铁对其结构和形貌影响条件的探究[J].湖北大学学报:自然科学版,2020,42(2):222-227,232.
|
|
KE Wang, JIA Yanmei, YU Xuefeng,et al.Study on the effects of multiple methods to prepare ferrous oxalate on its structure and morphology[J].Journal of Hubei University:Natural Science,2020,42(2):222-227,232.
|
| 13 |
HU Pengcheng, ZHANG Yimin, LIU Tao,et al.Separation and recovery of iron impurity from a vanadium-bearing stone coal via an oxalic acid leaching-reduction precipitation process[J].Separation and Purification Technology,2017,180:99-106.
|
 ),LI Xi2,ZHANG Jun3,DUAN Siyu1
),LI Xi2,ZHANG Jun3,DUAN Siyu1