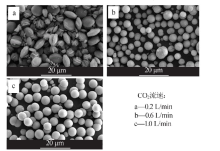
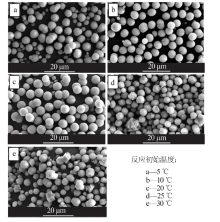
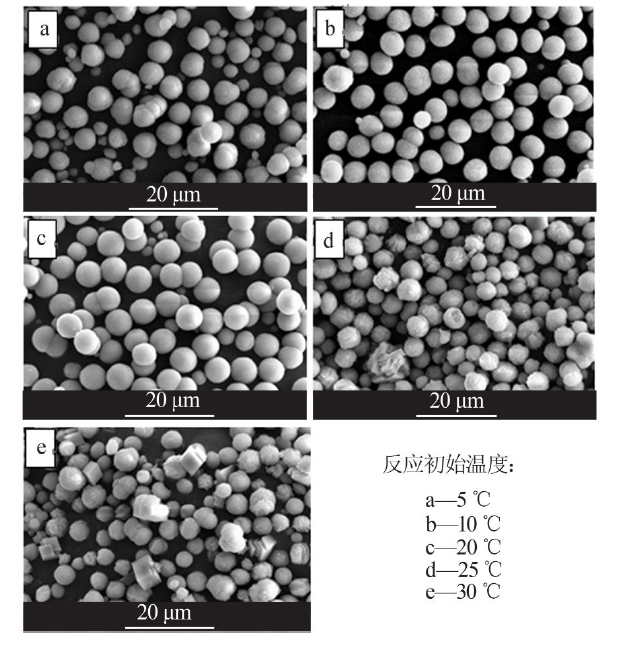
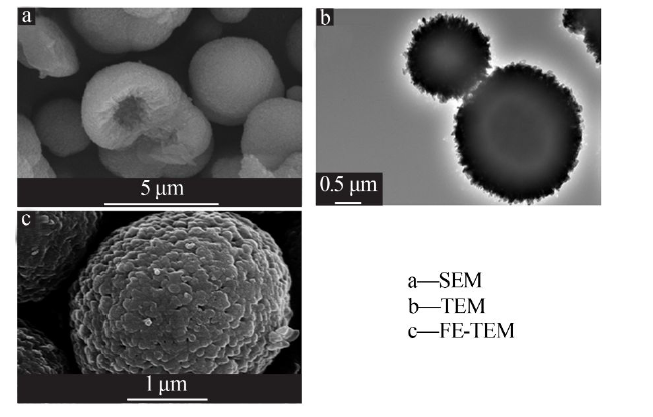
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (3): 38-43.doi: 10.11962/1006-4990.2020-0230
• Research & Development • Previous Articles Next Articles
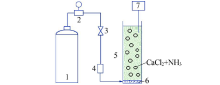
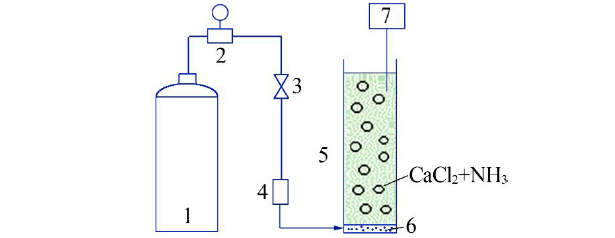
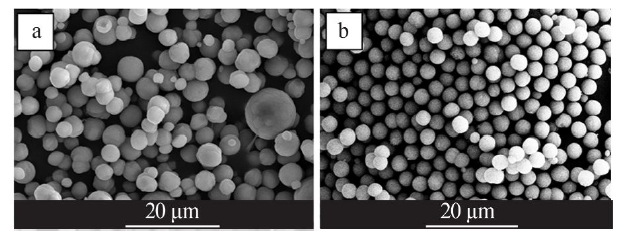
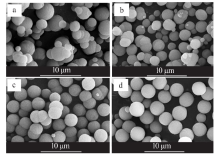
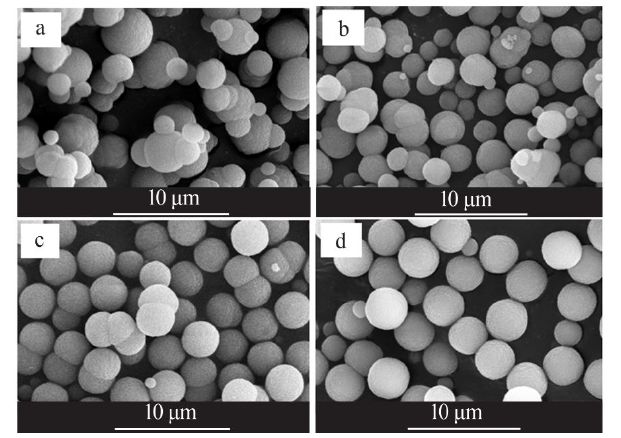
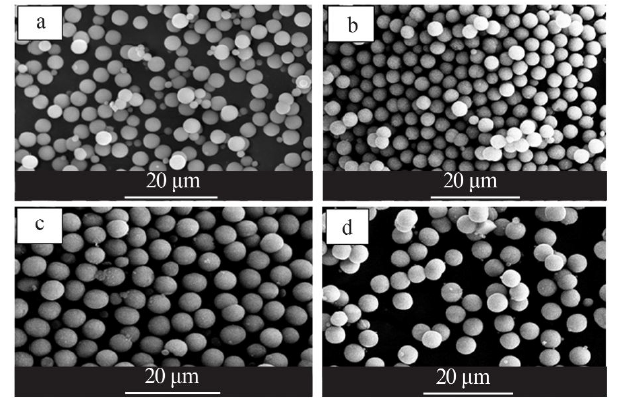
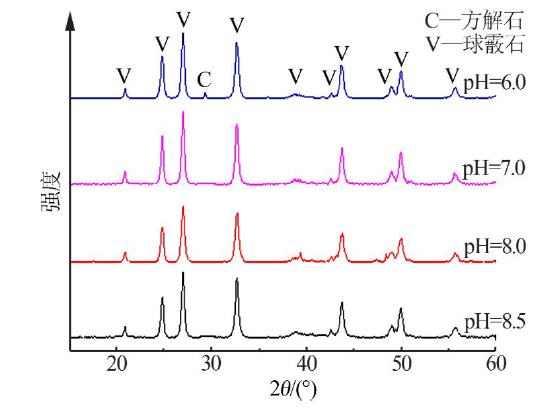
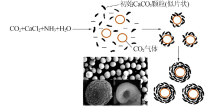
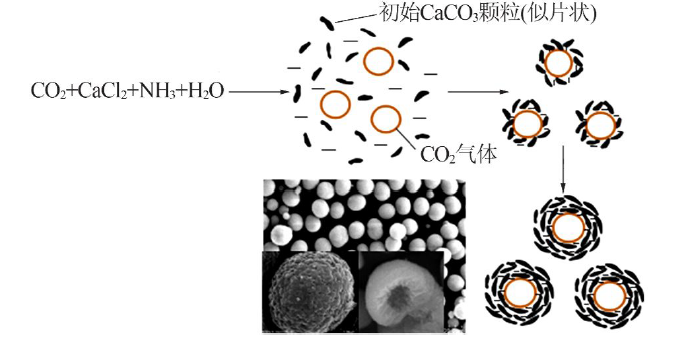
Synthesis of vaterite CaCO3 microspheres by carbonization method and its formation mechanism
Zhao Li1( ),Zhuo Minquan2,Gong Fuzhong1(
),Zhuo Minquan2,Gong Fuzhong1( ),Wang Jun2,Ruan Heng2,Li Kaicheng3,Li Yanlin1
),Wang Jun2,Ruan Heng2,Li Kaicheng3,Li Yanlin1
- 1. College of Chemistry and Chemical Engineering,Guangxi University,Nanning 530004,China
2. Guangxi Research Institute of Chemical Industry Co.,Ltd.
3. Guangxi Xinjing Technology Co.,Ltd.
-
Received:2020-09-18Online:2021-03-10Published:2021-03-11 -
Contact:Gong Fuzhong E-mail:zhaolimm@163.com;fzgong@gxu.edu.cn
CLC Number:
Cite this article
Zhao Li,Zhuo Minquan,Gong Fuzhong,Wang Jun,Ruan Heng,Li Kaicheng,Li Yanlin. Synthesis of vaterite CaCO3 microspheres by carbonization method and its formation mechanism[J]. Inorganic Chemicals Industry, 2021, 53(3): 38-43.
share this article
| [1] | Jimoh O A, Ariffin K S, Hussin H B, et al. Synjournal of precipitated calcium carbonate:a review[J]. Carbonates and Evaporites, 2018,33(2):331-346. |
| [2] | Trushina D B, Bukreeva T V, Kovalchuk M V, et al. CaCO3 vaterite microparticles for biomedical and personal care application[J]. Materials Science & Engineering:C, 2014,45(12):644-658. |
| [3] | Konopacka-Łyskawa D. Synjournal methods and favorable conditions for spherical vaterite precipitation:a review[J]. Crystals, 2019,9(4):2-16. |
| [4] | 蒋久信, 吴月, 何瑶, 等. 亚稳态球霰石相碳酸钙的调控制备进展[J]. 无机材料学报, 2017,32(7):681-690. |
| [5] |
Huang S C, Naka K, Chujo Y. A carbonate controlled-addition method for amorphous calcium carbonate spheres stabilized by poly (acrylic acid)s[J]. Langmuir, 2007,23(24):12086-12095.
pmid: 17963412 |
| [6] | Lai Y H, Chen L S, Bao W C, et al. Glycine-mediated,selective preparation of monodisperse spherical vaterite calcium carbonate in various reaction systems[J]. Crystal Growth & Design, 2015,15(3):1194-1200. |
| [7] | Daria B T, Tatiana V B, Maria N A. Size-controlled synjournal of vaterite calcium carbonate by the mixing method:Aiming for nanosized particles[J]. Crystal Growth & Design, 2016,16(3):1311-1319. |
| [8] | Pérez-Villarejo L, Takabait F, Mahtout L, et al. Synjournal of vaterite CaCO3 as submicron and nanosized particles using inorganic precursors and sucrose in aqueous medium[J]. Ceramics International, 2018,44(5):5291-5296. |
| [9] | 赵丽娜, 孔治国, 王继库. 碳酸钙中空微球的制备及机理[J]. 化工学报, 2012,43(6):1976-1980. |
| [10] | 郑天文, 陈雪梅. 球霰石碳酸钙微球的合成及其机理[J]. 材料科学与工程学报, 2018,36(3):258-264. |
| [11] | 王耀宣, 袁爱群, 周泽广, 等. 球霰石型纳米碳酸钙椭球形颗粒的合成[J]. 无机盐工业, 2020,52(1):54-58. |
| [12] | 谭婷婷, 仲剑初. 球形碳酸钙的控制合成研究[J]. 无机盐工业, 2019,51(12):30-34. |
| [13] | 陈银霞, 纪献兵, 景长勇. 花状球霰石碳酸钙微球的简易合成与表征[J]. 无机盐工业, 2018,50(3):28-30. |
| [14] | 张晓蕾, 邱勇波. 球霰石碳酸钙的制备及其稳定性研究[J]. 无机盐工业, 2018,50(2):46-49. |
| [15] | 丁杨, 任洋洋, 王丹, 等. 微乳液体系中形貌可控合成碳酸钙的研究[J]. 无机盐工业, 2017,49(6):33-36. |
| [16] | 黄文艺, 马蓝宇, 程昊, 等. 球霰石型碳酸钙微球的制备及在不同溶液中转变过程的研究[J]. 无机盐工业, 2017,49(5):18-21. |
| [17] | Udrea I, Capat C, Olaru E A, et al. Vaterite synjournal via gas-liquid route under controlled pH conditions[J]. Industrial & Engineering Chemistry Research, 2012,51(24):8185-8193. |
| [18] | Hadiko G, Han Y S, Fuji M, et al. Synjournal of hollow calcium carbonate particles by the bubble templating method[J]. Materials Letters, 2005,59(19/20):2519-2522. |
| [19] | Boyjoo Y, Pareek V K, Liu J. Synjournal of micro and nano-sized calcium carbonate particles and their applications[J]. Materials Chemistry A, 2014,2(35):14270-14288. |
| [20] | Konopacka-Łyskawa D, Koscielska B, Karczewski J, et al. The influence of ammonia and selected amines on the characteristics of calcium carbonate precipitated from calcium chloride solutions via carbonation[J]. Materials Chemistry and Physics, 2017,1931(6):13-18. |
| [21] | 王芬, 余军霞, 肖春桥, 等. CO2 碳化法制备微米级球霰石型食品碳酸钙的研究[J]. 硅酸盐通报, 2017,36(1):43-50. |
| [22] | Svenskaya Y I, Fattah H, Inozemtseva O A, et al. Key parameters for size and shape-controlled synjournal of vaterite particle[J]. Crystal Growth & Design, 2018,18(1):331-337. |
| [23] | Oral C M, Ercan B. Influence of pH on morphology,size and polymorph of room temperature synthesized calcium carbonate particles[J]. Powder Technology, 2018,339(11):781-788. |
| [24] | Watanabe H, Yoshiaki M, Takeshi E, et al. Effect of initial pH on formation of hollow calcium carbonate particles by continuous CO2 gas bubbling into CaCl2 aqueous solution[J]. Advanced Powder Technology, 2009,20(1):89-93. |
| [25] | Han Y S, Hadiko G, Fuji M, et al. Effect of flow rate and CO2 content on the phase and morphology of CaCO3 prepared by bubbling method[J]. Crystal Growth, 2005,276(3/4):541-548. |
| [26] | Han Y S, Fuji M, Shehukin D, et al. A new model for the synjournal of hollow particles via the bubble templating method[J]. Crystal Growth & Design, 2009,9(8):3771-3775. |
| [27] | Radek S, Pérez-Estébanez M, Viani A, et al. Characterization of vaterite synthesized at sized at various temperatures and stirring velocities without use of additives[J]. Powder Technology, 2015,284(11):265-271. |
| [28] | 向乐凯, 李枫, 赵宁, 等. 二氧化碳鼓泡碳化法制备碳酸钙的研究[J]. 无机盐工业, 2016,48(8):46-51. |
| [29] | Rodriguez-Blanco J D, Shaw S, Bots P, et al. The role of pH and Mg on the stability and crystallization of amorphous calcium carbonate[J]. Journal of Alloys & Compounds, 2012,536(supp_S1), S477-S479. |
| [30] | Sun J, Wang L S, Zhao D F. Polymorph and morphology of CaCO3 in relation to precipitation conditions in a bubbling system[J]. Chinese Journal of Chemical Engineering, 2017,25(9):1335-1342. |
| [31] | Takeshi O, Toshio S, Kiyoshi S. The formation and transformation mechanism of calcium carbonate in water[J]. Geochimica et Cosmochimica Acta, 1987,51(10):2757-2767. |
| [32] | Zeng Y P, Cao J, Wang Z, et al. The formation of amorphous calcium carbonate and its transformation mechanism to crystalline CaCO3 in laminar microfluidics[J]. Crystal Growth & Design, 2018,18(3):1710-1721. |
| [33] | Ding Y, Liu Y Y, Ren Y Y, et al. Controllable synjournal of all the anhydrous CaCO3 Polymorphs with various morphologies in CaCl2NH3-CO2 aqueous system[J]. Powder Technology, 2018,333(6):410-420. |
| [34] | Wang Y S, Moo Y X, Chen C P, et al. Fast precipitation of uniform CaCO3 nanospheres and their transformation to hollow hydroxyap-atite nanospheres[J]. Colloid and Interface Science, 2010,352(2):393-400. |
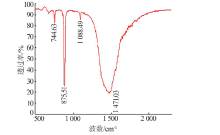
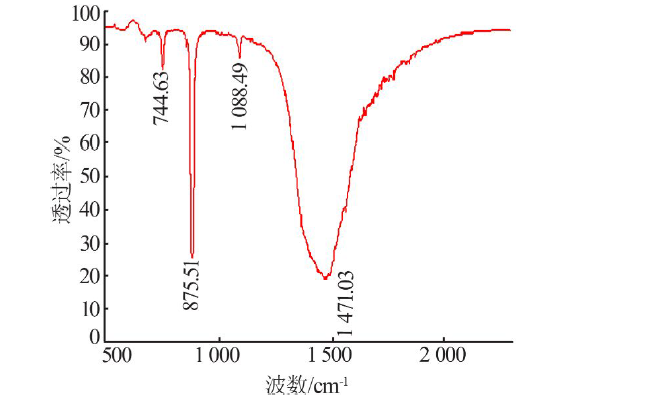
| [35] |
Vagenas N V, Gatsouli A, Kontoyannis C G. Quantitative analysis of synthetic calcium carbonate polymorphs using FT-IR spectro-scopy[J]. Talanta, 2003,59(4):831-836.
doi: 10.1016/S0039-9140(02)00638-0 pmid: 18968970 |
| [36] | Han Y S, Hadiko G, Fuji M, et al. Crystallization and transforma-tion of vaterite at controlled pH[J]. Journal of Crystal Growth, 2006,289(1):269-274. |
| [1] | LI Zihan, ZHANG Jiaqi, LI Shizhuo, LI Xinyu, LIU Shaozhuo, WANG Yihao, HAO Yucui, LIU Jian, LI Yanhua. Study on synthesis and catalytic mechanism of CdS/g-C3N4 composite photocatalyst [J]. Inorganic Chemicals Industry, 2025, 57(3): 124-132. |
| [2] | MA Jun, JIN Yang, LI Jun, CHEN Ming, WANG Yubin. Study on photochemical synthesis of H2O2 in coiled flow inverter microreactor [J]. Inorganic Chemicals Industry, 2025, 57(2): 50-56. |
| [3] | LIU Huangfei, ZHANG Li, LIU Tao. Research progress of fast synthesis technologies of zeolites [J]. Inorganic Chemicals Industry, 2025, 57(2): 36-43. |
| [4] | SU Baocai, ZHANG Qin, XIE Yuanjian, CAI Pingxiong, PAN Yuanfeng. Advances in synthesis methods and structural modification of LiMnFePO4 materials [J]. Inorganic Chemicals Industry, 2024, 56(7): 28-36. |
| [5] | HU Cheng, LIU Meng, XIANG Weiheng, DUAN Pengxuan, LI Shunkai, MING Yang, WANG Neng, LU Guanju. Effect of NaCl solution concentration on transcrystallization behavior of α-hemihydrate gypsum from phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(6): 87-93. |
| [6] | LI Yuxing, ZHANG Jincai, CHENG Fangqin. Research progress of preparation and growth mechanism of various crystalline nano-calcium carbonate [J]. Inorganic Chemicals Industry, 2024, 56(5): 1-10. |
| [7] | ZHANG Heng, CHEN Kunfeng, XUE Dongfeng. Research progress of high purity tantalum niobium [J]. Inorganic Chemicals Industry, 2024, 56(12): 1-12. |
| [8] | WANG Benlei, ZHANG Guisheng, JIANG Lingyun, LI Chen, WANG Pengfei, LI Jixia, ZANG Jiazhong. Study on preparation process of rhodium trichloride by roasting recovery technology from waste rhodium solution of carbonyl synthesis [J]. Inorganic Chemicals Industry, 2023, 55(6): 104-108. |
| [9] | HONG Meihua, GUO Zifeng, LIU Guanfeng, ZANG Jiazhong, YANG Keyu, YU Yonghua, ZHANG Dazhi, HUANG Shengjun. Progress and challenges of alkaline treatment for synthesis of hierarchical zeolites [J]. Inorganic Chemicals Industry, 2023, 55(6): 36-42. |
| [10] | LI Tong, YIN Hongfeng. Study on controllable preparation of nickel phyllosilicate nanotubes catalysts and their catalytic performance [J]. Inorganic Chemicals Industry, 2023, 55(5): 128-136. |
| [11] | DING Hongyu, ZHONG Jianchu, WANG Hongzhi, ZHANG Shuang. Hydrothermal synthesis of talc by crystal seed induction method [J]. Inorganic Chemicals Industry, 2023, 55(5): 59-65. |
| [12] | TAN Ying, LI Chongyi, YU Xiaoguang, LIAO Hongguang, YAN Xin. Bimetal doped SBA-15 catalytic synthesis of bisphenol F and its isomers regulation [J]. Inorganic Chemicals Industry, 2023, 55(11): 147-152. |
| [13] | YANG Tinglong,WANG Fuzhong,LIU Fei. Study on sulfur poisoning of zirconium-based bimetallic oxides catalyst [J]. Inorganic Chemicals Industry, 2023, 55(1): 151-158. |
| [14] | NAN Jinjian,WU Suli. Preparation of uniform cadmium sulfide microspheres in aqueous solution and their application in structural color [J]. Inorganic Chemicals Industry, 2022, 54(4): 94-99. |
| [15] | ZHANG Xinyi,DI Yuli,DONG Qi,CHEN Xingyu,ZHANG Zhengdong. Research progress on preparation of Li3V2(PO4)3 cathode material for lithium-ion batteries [J]. Inorganic Chemicals Industry, 2022, 54(3): 38-44. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||