Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (5): 70-73.
• Chemical Analysis & Testing • Previous Articles Next Articles
Determination and correlation of solubility of boric acid in calcium chloride solution
Chen Lifang1,2,Zhang Qinqin1,2,Lin Tian1,Yue Peiying1
- 1. College of Chemical Engineering and Materials,Tianjin University of Science & Technology,Tianjin 300457,China
2. Tianjin Key Laboratory of Marine Resources and Chemistry
-
Received:2018-11-11Online:2019-05-10Published:2020-04-28
CLC Number:
Cite this article
Chen Lifang,Zhang Qinqin,Lin Tian,Yue Peiying. Determination and correlation of solubility of boric acid in calcium chloride solution[J]. Inorganic Chemicals Industry, 2019, 51(5): 70-73.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
"
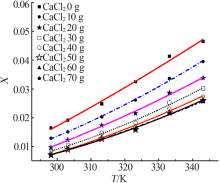
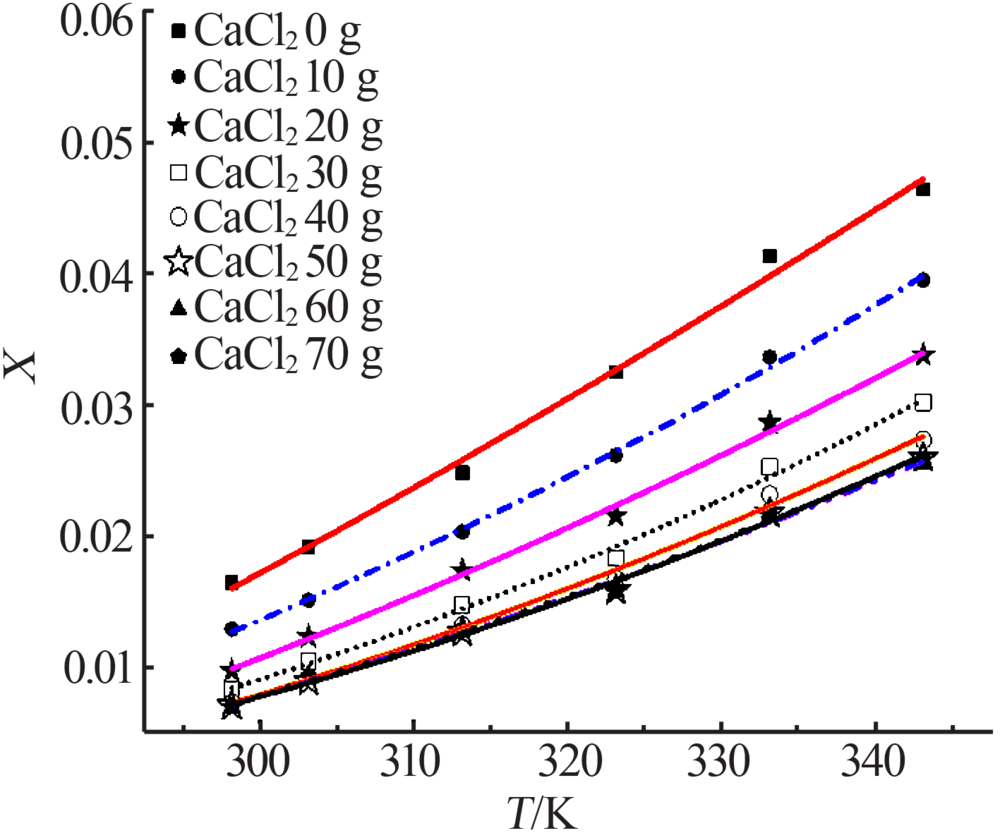
| m(CaCl2)/g | Apelblat 模型 | 经验模型 | λh模型 | |||||
|---|---|---|---|---|---|---|---|---|
| A1 | B1 | C1 | A2 | B2 | C2 | λ | h | |
| 0 | 119.975 6 | -7 865.620 4 | -17.148 3 | 1.353 2×10-6 | -1.746 4×10-4 | -0.052 2 | 0.126 2 | 15 141.325 6 |
| 10 | 101.001 1 | -7 110.889 7 | -14.301 9 | 2.706 0×10-6 | -1.130 0×10-3 | 0.109 9 | 0.125 4 | 16 814.115 6 |
| 20 | 164.139 3 | -10 262.878 0 | -23.572 9 | 1.830 8×10-6 | -6.386 8×10-4 | 0.037 6 | 0.124 7 | 18 359.444 6 |
| 30 | 57.434 5 | -5 304.478 2 | -7.787 7 | 2.866 8×10-6 | -1.400 0×10-3 | 0.156 1 | 0.127 7 | 19 290.123 6 |
| 40 | 133.819 2 | -9 012.366 1 | -19.039 1 | 2.254 2×10-6 | -9.935 5×10-4 | 0.103 1 | 0.123 1 | 20 599.578 0 |
| 50 | 77.710 9 | -6 288.041 3 | -10.795 9 | 2.353 8×10-6 | -1.100 0×10-3 | 0.122 2 | 0.109 8 | 22 458.505 3 |
| 60 | 79.980 2 | -6 336.936 5 | -11.161 3 | 1.668 4×10-6 | -6.575 3×10-4 | 0.054 9 | 0.101 2 | 23 552.583 9 |
| 70 | 61.347 0 | -5 456.688 4 | -8.406 7 | 1.883 1×10-6 | -7.8985×10-4 | 0.075 4 | 0.103 6 | 23 136.627 1 |
"
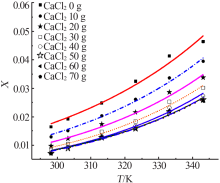
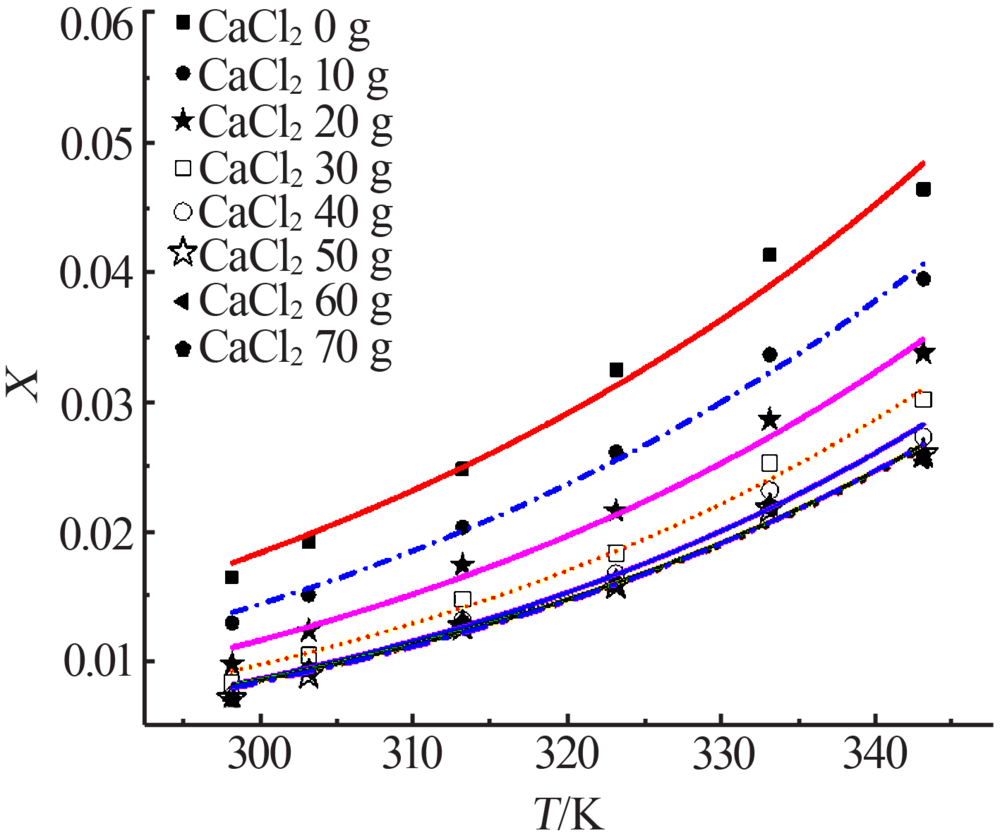
| m(CaCl2)/g | Apelblat 模型 | 经验模型 | λh 模型 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | ARD/% | RSMD/10-4 | R2 | ARD/% | RSMD/10-4 | R2 | ARD/% | RSMD/10-4 | |
| 0 | 0.992 1 | 1.709 1 | 9.830 1 | 0.994 4 | 2.092 2 | 8.275 7 | 0.982 7 | 4.040 6 | 14.556 0 |
| 10 | 0.998 1 | 1.112 9 | 4.155 9 | 0.998 4 | 4.023 3 | 9.448 2 | 0.991 7 | 3.404 2 | 8.750 8 |
| 20 | 0.995 3 | 2.648 4 | 5.882 7 | 0.997 2 | 1.817 0 | 4.554 3 | 0.987 4 | 4.945 1 | 9.551 7 |
| 30 | 0.993 9 | 3.151 2 | 6.089 5 | 0.995 7 | 2.310 0 | 5.101 5 | 0.990 0 | 3.973 5 | 8.109 5 |
| 40 | 0.993 3 | 2.615 6 | 5.886 4 | 0.995 7 | 1.857 9 | 4.716 7 | 0.987 2 | 4.924 6 | 8.147 6 |
| 50 | 0.994 6 | 2.919 1 | 4.947 5 | 0.996 4 | 6.064 0 | 8.822 7 | 0.989 9 | 4.339 0 | 6.739 2 |
| 60 | 0.991 9 | 4.095 5 | 5.926 9 | 0.994 6 | 2.861 3 | 4.846 3 | 0.985 3 | 5.090 2 | 7.966 7 |
| 70 | 0.990 6 | 4.418 1 | 6.455 8 | 0.993 4 | 3.215 5 | 5.430 3 | 0.985 1 | 5.041 6 | 8.124 1 |
| [1] | 郑学家 . 硼及硼酸盐产品开发和应用前景[J]. 无机盐工业, 2005,37(4):1-3. |
| [2] | 曲学孟 . 宽甸地区硼酸生产现状与展望[J]. 辽宁化工, 2001,30(7):313-314. |
| [3] | 熊妍 . 盐湖卤水萃取提硼技术研究[D]. 杭州:浙江大学, 2013. |
| [4] | 陈侠, 郑颖贺, 胡宇飞 , 等. 混合醇萃取深层地下卤水中硼的实验研究[J]. 无机盐工业, 2015,47(9):35-37. |
| [5] | 董乃金, 董亚萍, 彭姣玉 , 等. NaCl对H3BO3溶解度、界稳区宽度及成核动力学的影响[J]. 盐湖研究, 2015,23(3):29-36,51. |
| [6] | 龚殿婷, 李凤华, 樊占国 , 等. H3BO3-Na2SO4-H2O体系结晶动力学研究[J]. 郑州大学学报:工学版, 2009,30(1):82-86. |
| [7] | 三元体系H3BO3-MgCl2-H2O在308.15 K和323.15 K稳定相平衡研究[J]. 广东微量元素科学, 2012,19(6):46-51. |
| [8] | 李丽丽, 董亚萍, 彭姣玉 , 等. 硫酸镁对硼酸介稳区性质的影响[J]. 盐湖研究, 2013,21(1):38-43. |
| [9] | 胡程耀, 黄培 . 固体溶解度测定方法的近期研究进展[J]. 药物分析杂志, 2010,30(4):761-766. |
| [10] | Apelblat A, Manzurola E . Solubilities of o-acetylsalicylic,4-amino-salicylic,3,5-dinih dsalicylic,and p-toluicacid,and magnesium-DL-aspartate in water from T=(278 to 348) K[J]. J.Chem.Thermodyn., 1999,3l(10):85-91. |
| [11] | 李逢玲, 牛艳霞, 凌开成 , 等. 单质硫在不同溶剂中溶解度的测定与关联[J]. 科技情报开发与经济, 2011,21(5):184-187. |
| [12] |
Buchowski H, Ksiazczak A, Pietrzyk S . Solvent activity along a saturation line and solubility of hydrogen-bonding solids[J]. J.Phys. Chem., 1980,84(9):975-979.
doi: 10.1021/j100446a008 |
| [13] | Dean J A . 兰氏化学手册第十章[M]. 北京:科学出版社, 1991: 9. |
| [1] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| [2] | Liu Juexin,Zheng Chenggang,Ye Shichao. Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal [J]. Inorganic Chemicals Industry, 2021, 53(1): 62-64. |
| [3] | Wang Xiaoxiong,Zhang Jiangang,Zhang Wenjing,Chen Ye. Removal of calcium chloride from crude phosphoric acid by hydrochloric acid method [J]. Inorganic Chemicals Industry, 2020, 52(8): 17-19. |
| [4] | Zhang Liyuan,Wang Gang,Qi Meiling,Xie Yulong. Effect of surfactants on metastable zone and induction period of KCl crystals in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(6): 46-49. |
| [5] | Wang Jukui,Dong Xingfeng,Zhao Dong,Wang Shiqiang,Guo Yafei,Deng Tianlong. Solid-liquid phase equilibria in quaternary system lithium borate-potassium borate-magnesium borate-water at 308.15 K [J]. Inorganic Chemicals Industry, 2020, 52(5): 27-30. |
| [6] | Qi Meiling,Wang Gang,Zhang Liyuan,Xie Yulong. Determination of KCl solubility,interfacial region and induction period in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(5): 45-49. |
| [7] | Wang Yubin,Wang Wangbo,Zhu Xinfeng,Xun Jingwen,Dang Weiben. Effect of different magnesium salts on solubility behavior of calcium carbonate under magnetization [J]. Inorganic Chemicals Industry, 2020, 52(3): 35-38. |
| [8] | Wu Qiang,Hu Xue,Zhu Jing,Yang Jiamin,Li Tianxiang. Phase equilibrium of KH2PO4-KCl-H2O and NH4H2PO4-NH4Cl-H2O ternary system at 283.15 K [J]. Inorganic Chemicals Industry, 2020, 52(11): 24-28. |
| [9] | Zhang Dongxue,Yang Baojun,Chen Yujia,Wang Bainian,Shao Zongqi. Preparation of calcium sulfate dihydrate whiskers from leachate of phosphate ore by hydrochloric acid [J]. Inorganic Chemicals Industry, 2020, 52(11): 37-42. |
| [10] | Bi Shengnan,Zhong Jianchu,Qi Ye,Ye Junwei,Ning Guiling. Latest research progress and industrialization trend of boron-containing chemicals such as boric acid [J]. Inorganic Chemicals Industry, 2020, 52(1): 5-8. |
| [11] | Cao Peng. Effect of acid soluble main reverse on solubility of solid phase of titanium white production by sulfuric acid process [J]. Inorganic Chemicals Industry, 2019, 51(9): 54-56. |
| [12] | Liu Leilei,Xia Haijian,Feng Weiliang,Wang Tielin,Wang Cunwen,Wang Weiguo,Jiang Zhensheng. Determination and correlation of solubility of potassium fluosilicate in dilute sulfuric acid solution [J]. Inorganic Chemicals Industry, 2019, 51(8): 17-19. |
| [13] | Zhang Wenhao,Yang Libin,Sha Zuoliang,Wang Yanfei,Zhu Liang,Zhao Xiaoyu. Determination of crystalline metastable zone and primary nucleationanalysis of lanthanum chloride heptahydrate [J]. Inorganic Chemicals Industry, 2019, 51(7): 43-47. |
| [14] | Yan Xin1,Lu Yunfeng2,Ma Yuanyuan3,Xie Long3. Study on composite carbonization mechanism of nano calcium carbonateproduced by calcium chloride-ammonia water system [J]. Inorganic Chemicals Industry, 2019, 51(7): 77-80. |
| [15] | Zhang Chan,Cheng Wenting,Cheng Huaigang,Guo Yanxia,Cheng Fangqin. Simulation and determination of AlCl3·6H2O solubility in FeCl3-CaCl2-HCl-H2O system [J]. Inorganic Chemicals Industry, 2019, 51(5): 61-65. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|