Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (11): 24-28.doi: 10.11962/1006-4990.2019-0639
• Research & Development • Previous Articles Next Articles
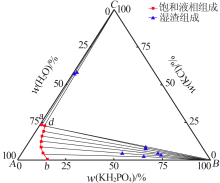
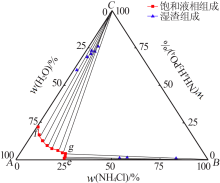
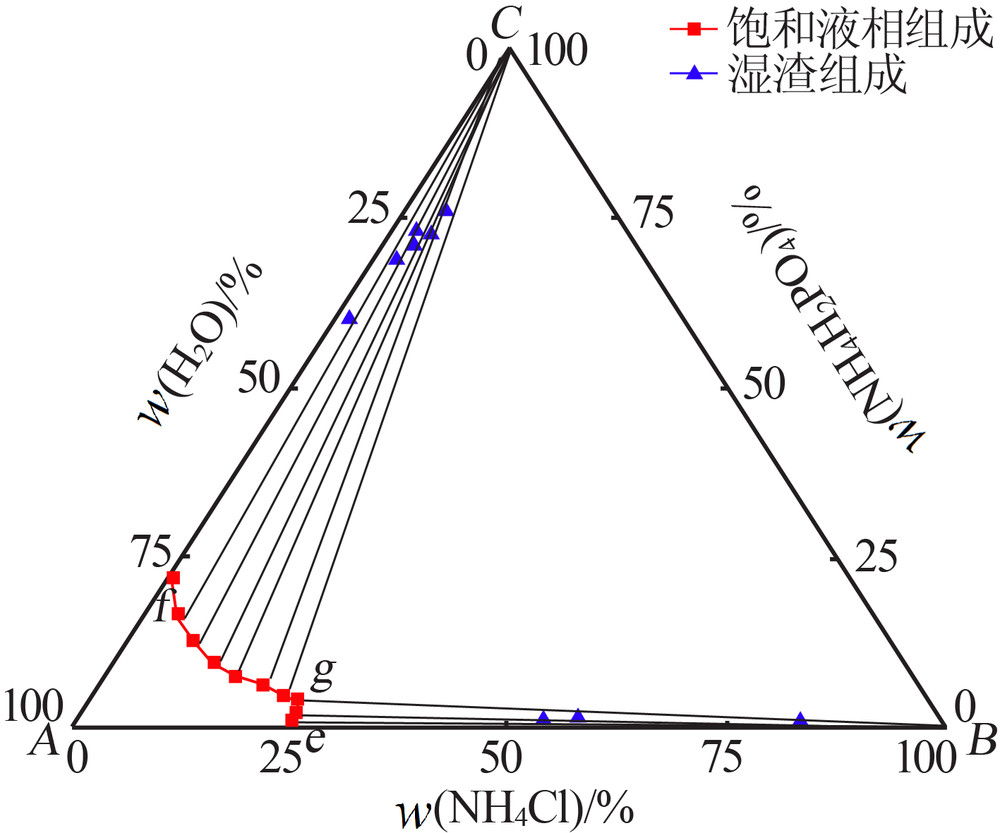
Phase equilibrium of KH2PO4-KCl-H2O and NH4H2PO4-NH4Cl-H2O ternary system at 283.15 K
Wu Qiang( ),Hu Xue,Zhu Jing,Yang Jiamin,Li Tianxiang(
),Hu Xue,Zhu Jing,Yang Jiamin,Li Tianxiang( )
)
- School of Chemistry and Chemical Engineering,Guizhou University,Guiyang 550025,China
-
Received:2020-05-16Online:2020-11-10Published:2020-12-01 -
Contact:Li Tianxiang E-mail:1483273451@qq.com;txli@gzu.edu.cn
CLC Number:
Cite this article
Wu Qiang,Hu Xue,Zhu Jing,Yang Jiamin,Li Tianxiang. Phase equilibrium of KH2PO4-KCl-H2O and NH4H2PO4-NH4Cl-H2O ternary system at 283.15 K[J]. Inorganic Chemicals Industry, 2020, 52(11): 24-28.
share this article
"
| 编号 | 液相组成/%(质量分数) | 湿渣组成/% (质量分数) | 固相 | ||||
|---|---|---|---|---|---|---|---|
| 实验值 | Pitzer计算值 | ||||||
| w (KH2PO4) | w (KCl) | w (KH2PO4) | w (KCl) | w (KH2PO4) | w (KCl) | ||
| 1(b) | 15.47 | 0.00 | 15.52 | 0.00 | — | — | KH2PO4 |
| 2 | 10.99 | 4.49 | 10.96 | 4.48 | 64.28 | 2.14 | KH2PO4 |
| 3 | 8.15 | 8.61 | 8.05 | 8.56 | 52.21 | 4.26 | KH2PO4 |
| 4 | 6.27 | 12.36 | 6.15 | 12.30 | 72.50 | 3.30 | KH2PO4 |
| 5 | 4.77 | 15.95 | 5.02 | 16.08 | 70.70 | 4.43 | KH2PO4 |
| 6 | 3.86 | 19.28 | 3.86 | 19.28 | 62.86 | 7.35 | KH2PO4 |
| 7 | 3.06 | 22.61 | 3.00 | 22.58 | 62.19 | 8.31 | KH2PO4 |
| 8(d) | 2.93 | 22.99 | 2.25 | 22.62 | 35.50 | 22.76 | KH2PO4+ KCl |
| 9 | 2.87 | 23.01 | 2.21 | 22.65 | 1.77 | 58.17 | KCl |
| 10 | 1.58 | 23.12 | 2.00 | 23.35 | 1.04 | 56.86 | KCl |
| 11(a) | 0.00 | 23.69 | 0.00 | 24.22 | — | — | KCl |
"
| 编号 | 液相组成/%(质量分数) | 湿渣组成/%(质量分数) | 固相 | ||||
|---|---|---|---|---|---|---|---|
| 实验值 | Pitzer计算值 | ||||||
| w(NH4Cl) | w(NH4H2PO4) | w(NH4Cl) | w(NH4H2PO4) | w(NH4Cl) | w(NH4H2PO4) | ||
| 1(e) | 24.98 | 0.00 | 25.47 | 0.00 | — | — | NH4Cl |
| 2 | 24.54 | 1.39 | 24.70 | 1.74 | 53.51 | 0.88 | NH4Cl |
| 3 | 24.31 | 2.84 | 24.05 | 2.28 | 57.02 | 1.57 | NH4Cl |
| 4 | 24.14 | 3.35 | 23.76 | 2.53 | 82.89 | 0.81 | NH4Cl |
| 5(g) | 23.64 | 4.47 | 23.64 | 4.48 | 10.51 | 61.23 | NH4Cl+ NH4H2PO4 |
| 6 | 21.63 | 5.04 | 21.66 | 5.11 | 4.76 | 76.05 | NH4H2PO4 |
| 7 | 18.61 | 6.10 | 18.61 | 6.09 | 4.84 | 72.45 | NH4H2PO4 |
| 8 | 14.98 | 7.49 | 15.07 | 7.69 | 4.02 | 70.66 | NH4H2PO4 |
| 9 | 11.52 | 9.60 | 11.43 | 9.41 | 2.91 | 72.95 | NH4H2PO4 |
| 10 | 7.58 | 12.69 | 7.41 | 12.33 | 2.63 | 68.95 | NH4H2PO4 |
| 11 | 3.78 | 16.89 | 3.63 | 16.57 | 1.66 | 60.15 | NH4H2PO4 |
| 12(f) | 0.00 | 22.01 | 0.00 | 22.60 | — | — | NH4H2PO4 |
| [1] | 周鹂, 鲁剑巍, 李小坤, 等. 我国大量元素水溶肥料产业发展现状[J]. 现代化工, 2013,33(4):9-14. |
| [2] | 张强, 付强强, 陈宏坤, 等. 我国水溶性肥料的发展现状及前景[J]. 山东化工, 2017,46(12):78-81. |
| [3] | 岳焕芳, 程明, 王俊英, 等. 水溶肥应用现状和发展前景[J].蔬菜, 2017(2):28-31. |
| [4] |
Gagnière E, Mangin D, Puel F, et al. Formation of co-crystals:Kine-tic and thermodynamic aspects[J]. Journal of Crystal Growth, 2009,311(9):2689-2695.
doi: 10.1016/j.jcrysgro.2009.02.040 |
| [5] | 冯先明, 王保明, 彭全, 等. 我国水溶肥的发展概况与建议[J]. 现代化工, 2018,38(1):6-11. |
| [6] |
Guo L, Hu Y, Wang Y, et al. A quasi-ternary wet residue method app-lied to solid-liquid equilibrium systems[J]. Fluid Phase Equilibria, 2018,456:161-167.
doi: 10.1016/j.fluid.2017.10.014 |
| [7] | 胡雪, 朱静, 王睿哲, 等.(NH2)2CO-NH4H2PO4-H2O三元体系10 ℃相平衡研究[J]. 无机盐工业, 2019,51(5):41-44. |
| [8] | Li G, Cao J, Chen P, et al. Determination and calculation of phase equilibrium for tetrahydrofuran+sodium sulfate+magnesium sulfate+water system at 5 ℃[J]. Journal of Chemical & Engineering Data, 2013,58(5):1301-1307. |
| [9] | 何贤江, 郭志琴, 苏裕光. 15 ℃ KCl-NaCl-MgCl2-H2O四元体系相平衡研究[J]. 化学学报, 1991,49:128-134. |
| [10] | 赵长伟, 马沛生, 郭瓦力, 等. KCl-NH4Cl-H2O 三元水盐体系溶解度的研究[J]. 化学工业与工程, 2003,20(3):145-149. |
| [11] | Pitzer K S. Thermodynamics of electrolytes.I.Theoretical basis and general equations[J]. The Journal of Physical Chemistry, 1973,77(2):268-277. |
| [12] | Silvester L F, Pitzer K S. Thermodynamics of electrolytes.8.High-temperature properties,including enthalpy and heat capacity,with application to sodium chloride[J]. The Journal of Physical Chemi-stry, 1977,81(19):1822-1828. |
| [13] | Pitzer K S, Peterson J R, Silvester L F. Thermodynamics of electro-lytes.IX.Rare earth chlorides,nitrates,and perchlorates[J]. Journal of Solution Chemistry, 1978,7(1):45-56. |
| [14] | Silvester L F, Pitzer K S. Thermodynamics of electrolytes.X.Enth-alpy and the effect of temperature on the activity coefficients[J]. Journal of Solution Chemistry, 1978,7(5):327-336. |
| [15] | Pitzer K S, Silvester L F. Thermodynamics of electrolytes.11.Pro-perties of 3:2,4:2,and other high-valence types[J]. The Journal of Physical Chemistry, 1978,82(11):1239-1242. |
| [16] | Bradley D J, Pitzer K S. Thermodynamics of electrolytes.12.Dielec-tric properties of water and debye-hiickel parameters to 350 ℃ and 1 kbar[J]. The Journal of Physical Chemistry, 1979,12(83):1599-1603. |
| [17] | Pitzer K S, Wang P, Rard J A, et al. Thermodynamics of electrolyt-es.13.Ionic strength dependence of higher-order terms;equations for CaCl2 and MgCl2[J]. Journal of Solution Chemistry, 1999,28(4):265-282. |
| [18] | 邓天龙, 周桓, 陈侠. 水盐体系相图及其应用[M]. 北京: 化学工业出版社, 2013. |
| [19] | Simoes M C, Hughes K J, Ingham D B, et al. Estimation of the pi-tzer parameters for 1-1,2-1,3-1,4-1,and 2-2 single electrolyt-es at 25 ℃[J]. Journal of Chemical & Engineering Data, 2016,61(7):2536-2554. |
| [1] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| [2] | Liu Juexin,Zheng Chenggang,Ye Shichao. Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal [J]. Inorganic Chemicals Industry, 2021, 53(1): 62-64. |
| [3] | Zhang Liyuan,Wang Gang,Qi Meiling,Xie Yulong. Effect of surfactants on metastable zone and induction period of KCl crystals in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(6): 46-49. |
| [4] | Wang Jukui,Dong Xingfeng,Zhao Dong,Wang Shiqiang,Guo Yafei,Deng Tianlong. Solid-liquid phase equilibria in quaternary system lithium borate-potassium borate-magnesium borate-water at 308.15 K [J]. Inorganic Chemicals Industry, 2020, 52(5): 27-30. |
| [5] | Qi Meiling,Wang Gang,Zhang Liyuan,Xie Yulong. Determination of KCl solubility,interfacial region and induction period in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(5): 45-49. |
| [6] | Wang Yubin,Wang Wangbo,Zhu Xinfeng,Xun Jingwen,Dang Weiben. Effect of different magnesium salts on solubility behavior of calcium carbonate under magnetization [J]. Inorganic Chemicals Industry, 2020, 52(3): 35-38. |
| [7] | Cao Peng. Effect of acid soluble main reverse on solubility of solid phase of titanium white production by sulfuric acid process [J]. Inorganic Chemicals Industry, 2019, 51(9): 54-56. |
| [8] | Liu Leilei,Xia Haijian,Feng Weiliang,Wang Tielin,Wang Cunwen,Wang Weiguo,Jiang Zhensheng. Determination and correlation of solubility of potassium fluosilicate in dilute sulfuric acid solution [J]. Inorganic Chemicals Industry, 2019, 51(8): 17-19. |
| [9] | Zhang Wenhao,Yang Libin,Sha Zuoliang,Wang Yanfei,Zhu Liang,Zhao Xiaoyu. Determination of crystalline metastable zone and primary nucleationanalysis of lanthanum chloride heptahydrate [J]. Inorganic Chemicals Industry, 2019, 51(7): 43-47. |
| [10] | Wu Linxin,Zeng Ying,Chen Peijun,Huang Peng,Chen Yu,Sun Jiu,Yu Xudong. Stable phase equilibrium of the ternary system K +,Cs +//SO4 2--H2O at 298.2 K [J]. Inorganic Chemicals Industry, 2019, 51(6): 17-20. |
| [11] | Hu Xue,Zhu Jing,Wang Ruizhe,Li Hongguo,Li Tianxiang. Research on phase equilibrium of ternary system of (NH2)2CO-NH4H2PO4-H2O at 10 ℃ [J]. Inorganic Chemicals Industry, 2019, 51(5): 41-44. |
| [12] | Zhang Chan,Cheng Wenting,Cheng Huaigang,Guo Yanxia,Cheng Fangqin. Simulation and determination of AlCl3·6H2O solubility in FeCl3-CaCl2-HCl-H2O system [J]. Inorganic Chemicals Industry, 2019, 51(5): 61-65. |
| [13] | Chen Lifang,Zhang Qinqin,Lin Tian,Yue Peiying. Determination and correlation of solubility of boric acid in calcium chloride solution [J]. Inorganic Chemicals Industry, 2019, 51(5): 70-73. |
| [14] | BI Si-Feng, CUI Xiang-Mei. Freezing conversion of brine in Yiliping salt lake [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 26-. |
| [15] | REN Xiao-Jing, HUANG Xue-Li. Phase equilibria in the quatemary system of Li+,Mg2+∥Cl-,SO42--H2O at 273.15 K [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 13-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|