Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (9): 54-56.doi: 10.11962/1006-4990.2018-0604
• Industrial Techniques • Previous Articles Next Articles
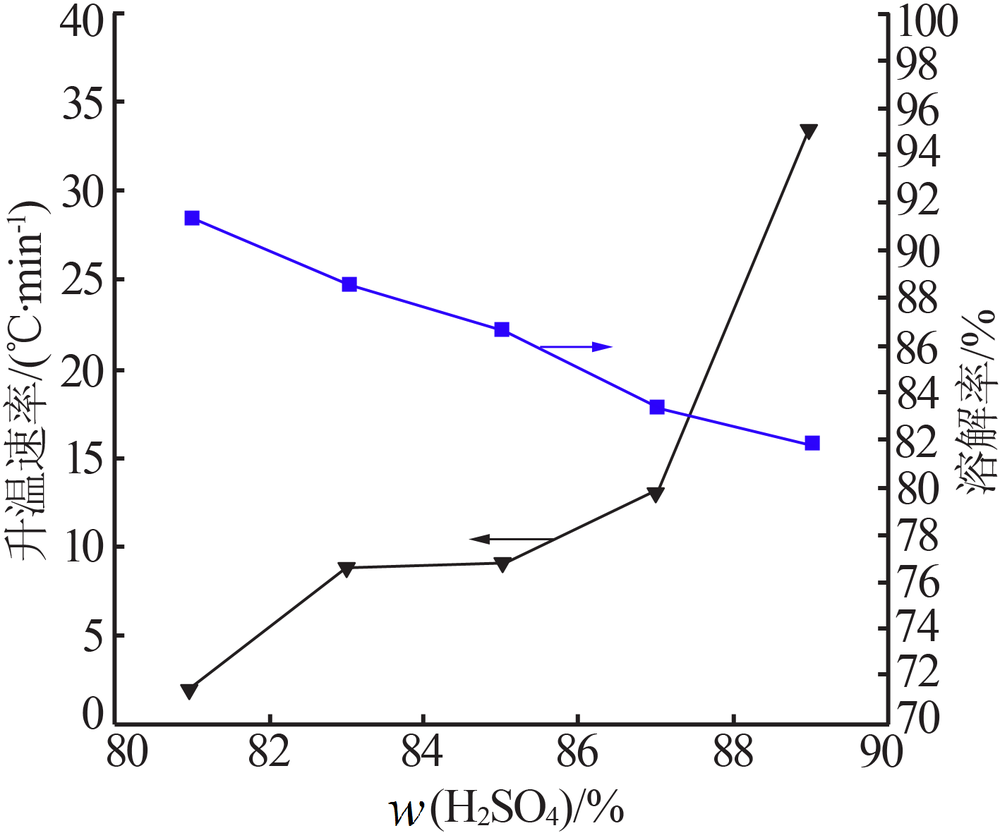
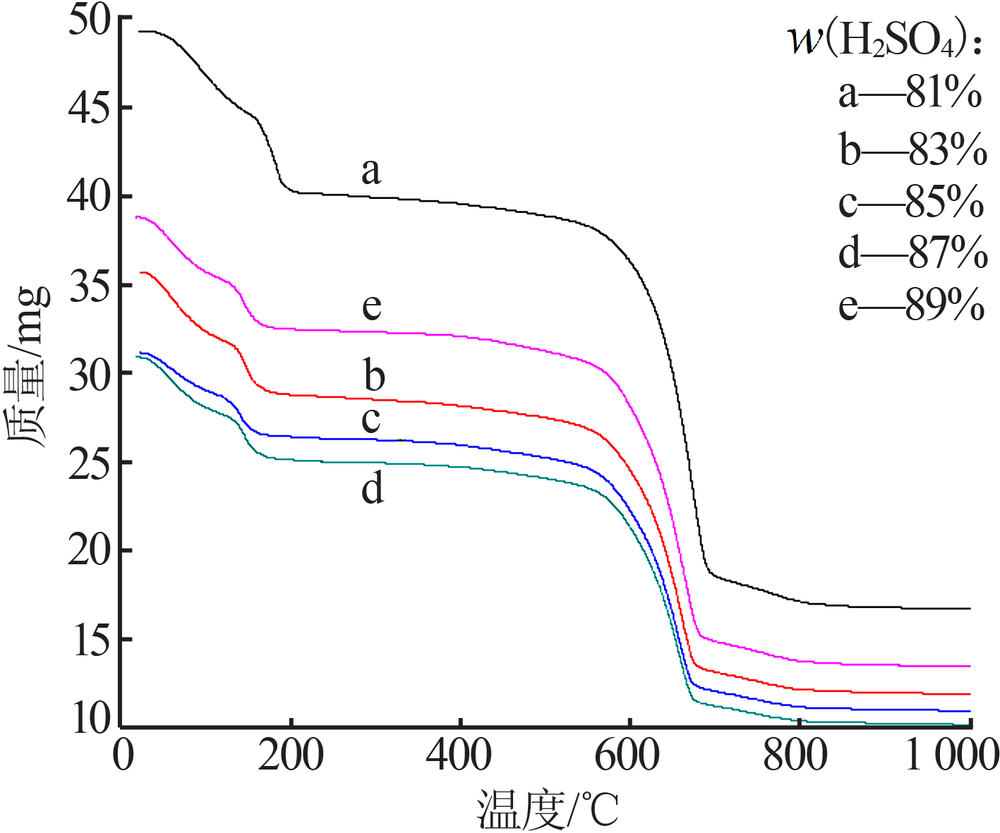
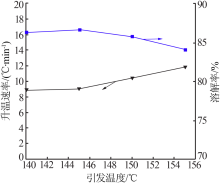
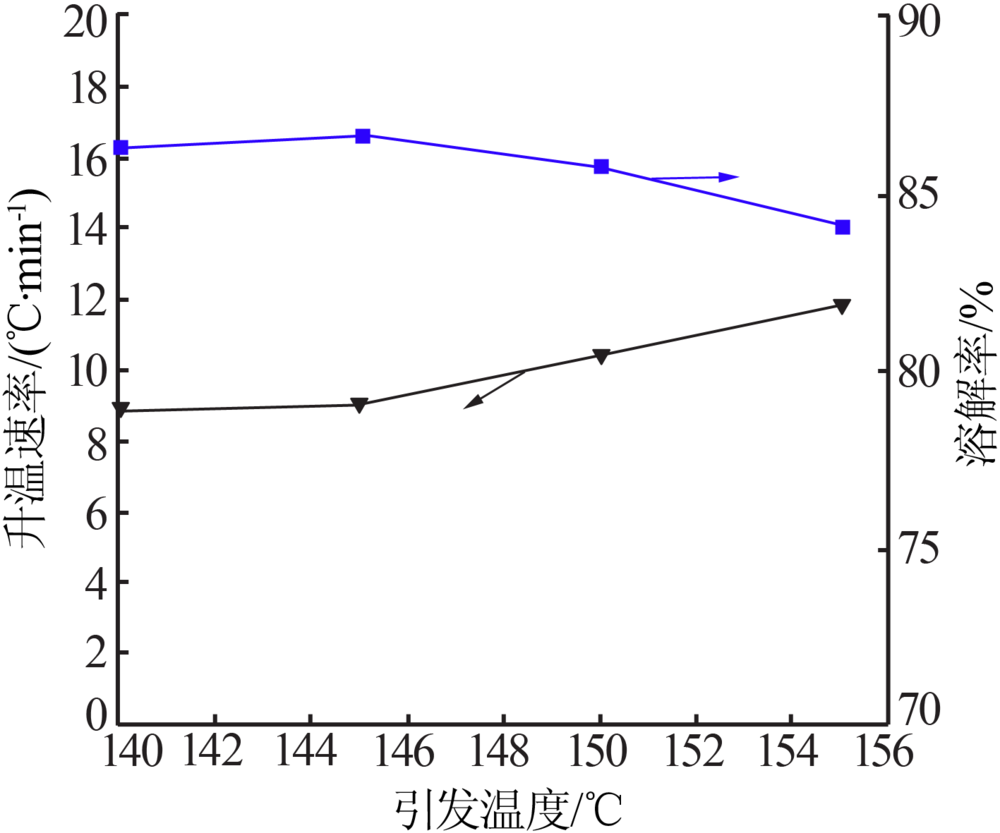
Effect of acid soluble main reverse on solubility of solid phase of titanium white production by sulfuric acid process
Cao Peng
- Sichuan Lomon Titanium Co.,Ltd.,Mianzhu 618200,China