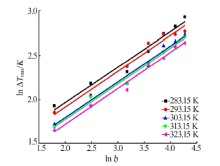
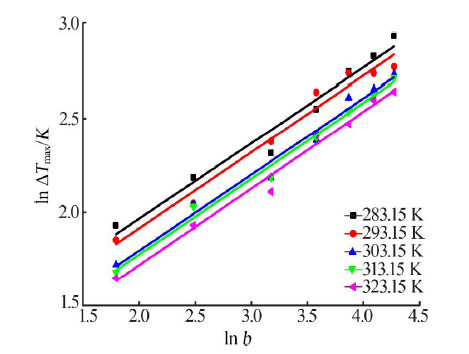
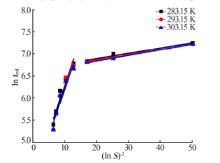
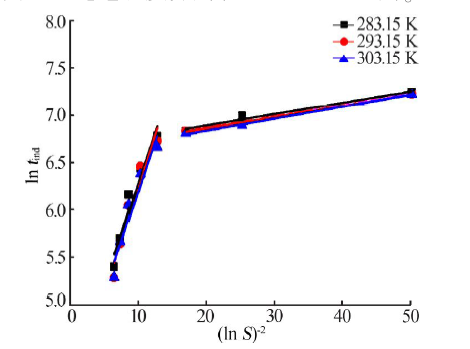
| [1] |
王兴富, 王石军, 田红斌 , 等. 青海盐湖提钾技术进展与我国钾肥工业的发展[J]. 化工矿物与加工, 2017(11):48-52.
|
| [2] |
Yong M A, Zhu Jiawen, Chen Kui , et al. Study on the properties of metastable zone of phosphoric acid crystal[J]. Journal of Chemical Engineering of Chinese Universities, 2010,24(2):331-335.
|
| [3] |
Lu Y H, Ching C B . Study on the metastable zone width of ketoprofen[J]. Chirality, 2010,18(4):239-244.
|
| [4] |
Kashchiev D, Borissova A, Hammond R B , et al. Effect of cooling rate on the critical undercooling for crystallization[J]. Journal of Crystal Growth, 2010,312(5):698-704.
|
| [5] |
Sangwal K, Mielniczek Brzoska E . Effect of impurities on metastable zone width for the growth of ammonium oxalate monohydrate cryst-als from aqueous solutions[J]. Journal of Crystal Growth, 2004,267(3/4):662-675.
|
| [6] |
Kadam S S, Kulkarni S A, Ribera R C , et al. A new view on the metastable zone width during cooling crystallization[J]. Chemical Engineering Science, 2012,72:10-19.
doi: 10.1016/j.ces.2012.01.002
|
| [7] |
Peng B, Zi J, Yan W . Measurement and correlation of solubilities of luteolin in organic solvents at different temperatures[J]. Journal of Chemical & Engineering Data, 2006,51(6):2038-2040.
|
| [8] |
李小娜 . 对苯二酚冷却结晶过程研究[D]. 天津:天津大学, 2006.
|
| [9] |
刘欣玉, 孙杰, 罗义芬 , 等. ADN的溶解度、结晶介稳区及诱导期的测定[J]. 含能材料, 2019(9).Doi: 10.11943/CJEM2018267.
|
| [10] |
Kubota Noriaki . A new interpretation of metastable zone widths me-asured for unseeded solutions[J]. Journal of Crystal Growth, 2008,310(3):629-634.
|
| [11] |
Nyvlt J . Kinetics of nucleation in solutions[J]. Journal of Crystal Growth, 1968,3/4:377-383.
doi: 10.1016/0022-0248(68)90179-6
|
| [12] |
Sangwal K . A novel self consistent Nyvlt like equation for metastable zone width determined by the polythermal method[J]. Crystal Research & Technology, 2009,44(4):231-247.
|
| [13] |
Mullin, J W . Crystallization[M]. 4th ed Oxford:Butterworth-Heine-mann, 2001: 206.
|
| [14] |
Ushasree P M, Muralidharan R, Jayavel R , et al. Metastable zone width,induction period and interfacial energy of zinc tris(thiourea) sulfate[J]. Journal of Crystal Growth, 2000,210(4):741-745.
doi: 10.1016/S0022-0248(99)00900-8
|
| [15] |
Jing D, Wang J, Zhang M , et al. Nucleation kinetics and growth model of penicillin sulfoxide in butyl acetate[J]. Chemical Engi-neering & Technology, 2013,36(10):1773-1778.
|
 ),Zhang Liyuan,Xie Yulong
),Zhang Liyuan,Xie Yulong