Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (3): 35-38.doi: 10.11962/1006-4990.2019-0268
• Research & Development • Previous Articles Next Articles
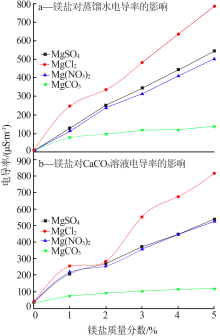
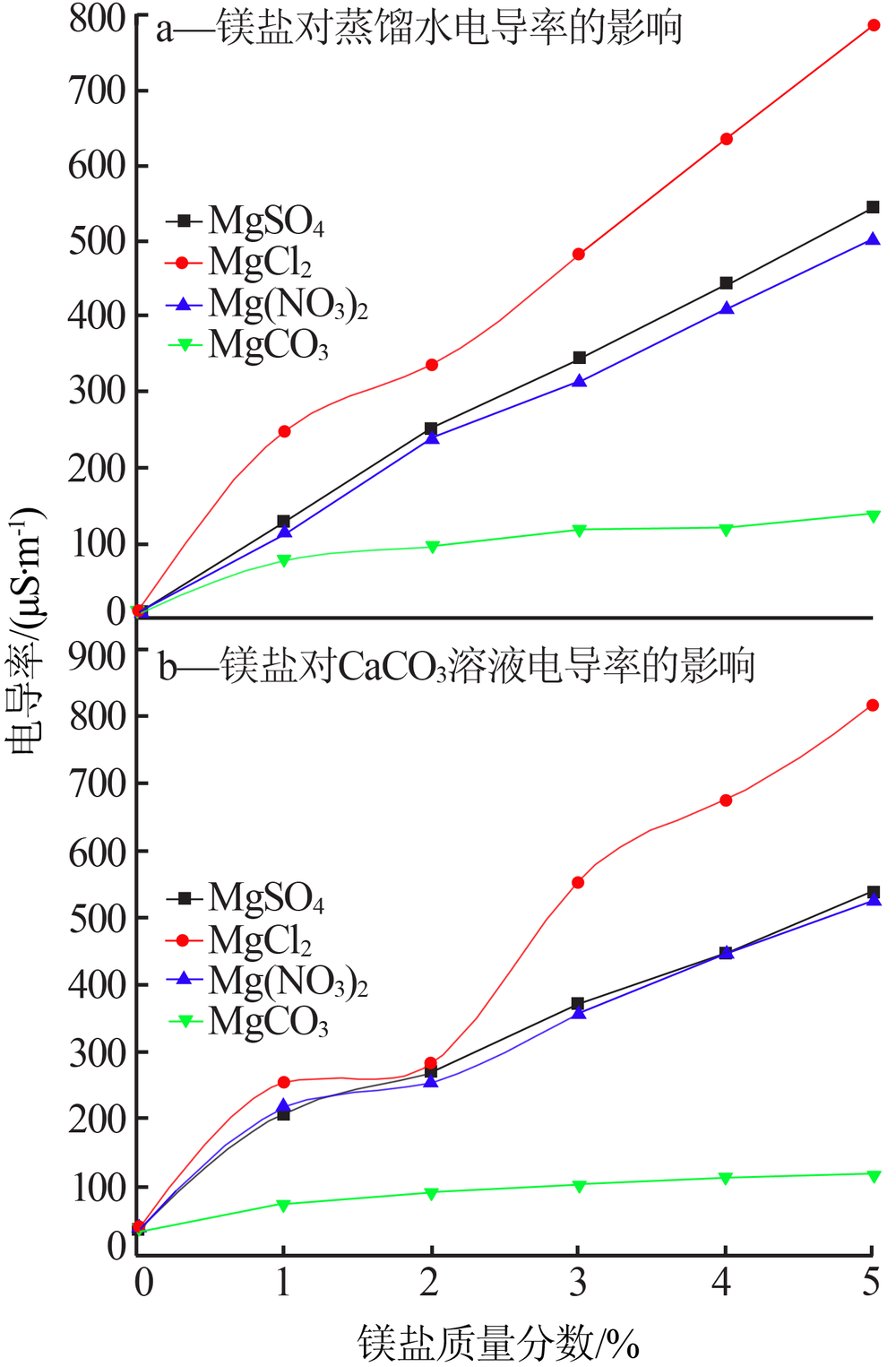
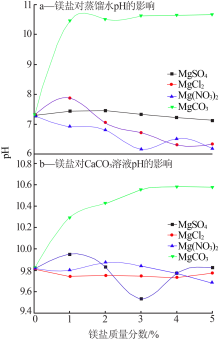
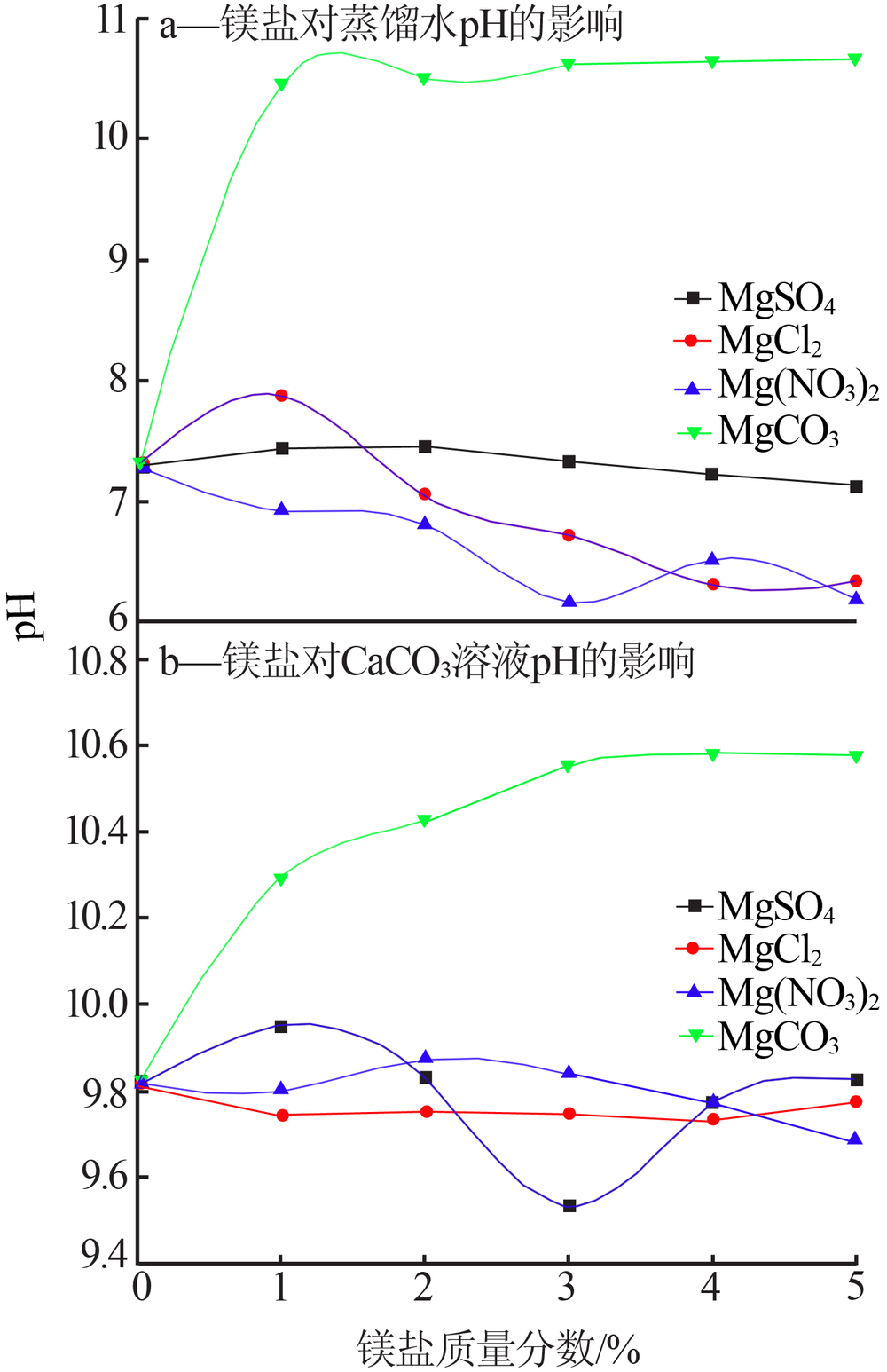
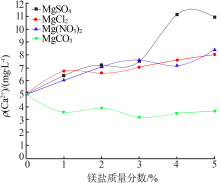
Effect of different magnesium salts on solubility behavior of calcium carbonate under magnetization
Wang Yubin1,2,Wang Wangbo1( ),Zhu Xinfeng2,Xun Jingwen1,Dang Weiben1
),Zhu Xinfeng2,Xun Jingwen1,Dang Weiben1
- 1. School of Resources Engineering,Xi′an University of Architecture and Technology,Xi′an 710055,China;
2. Henan Province Key Laboratory of Water Pollution Control and Rehabilitation