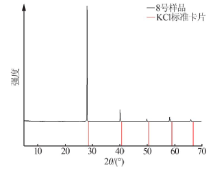
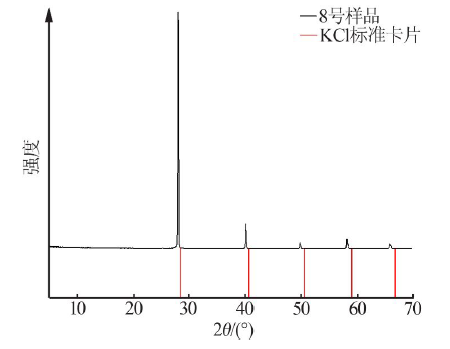
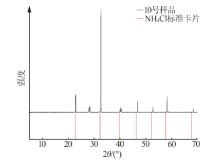
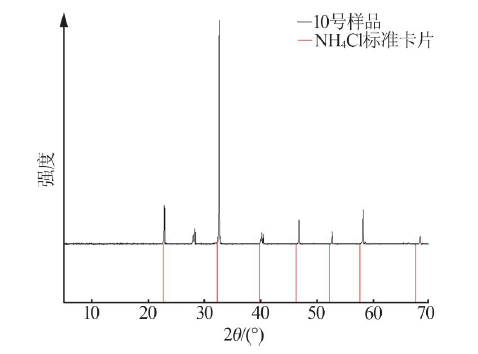
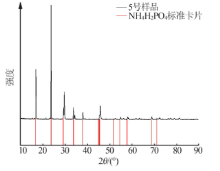
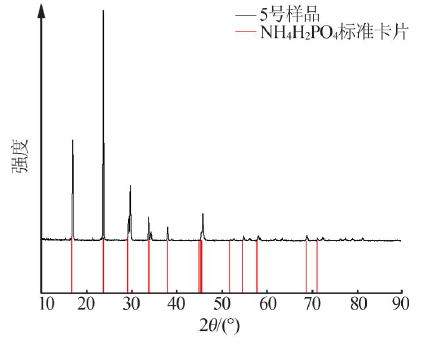
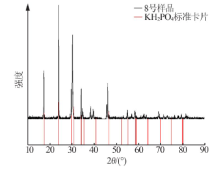
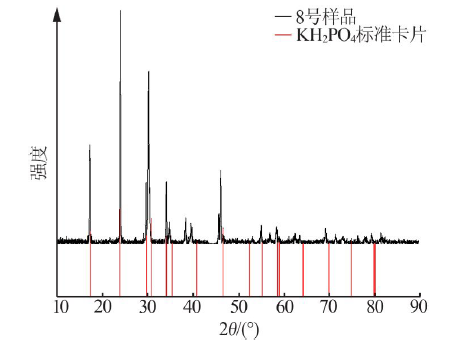
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (1): 30-35.doi: 10.11962/1006-4990.2020-0103
Previous Articles Next Articles
Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K
Yang Jiamin( ),Zhu Jing(
),Zhu Jing( ),Hu Xue,Wu Qiang,Li Tianxiang
),Hu Xue,Wu Qiang,Li Tianxiang
- School of Chemistry and Chemical Engineering,Guizhou University,Guiyang 550025,China
-
Received:2020-07-11Online:2021-01-10Published:2021-01-08 -
Contact:Zhu Jing E-mail:284226775@qq.com;jzhu1@gzu.edu.cn
CLC Number:
Cite this article
Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K[J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35.
share this article
"
| 序 号 | 液相组成/% | 湿固相/% | 平衡 固相 | ||||
|---|---|---|---|---|---|---|---|
| 实验值 | Pitzer计算值 | ||||||
| w (KCl) | w (NH4Cl) | w (KCl) | w (NH4Cl) | w (KCl) | w (NH4Cl) | ||
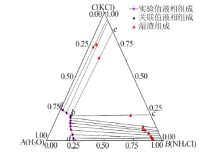
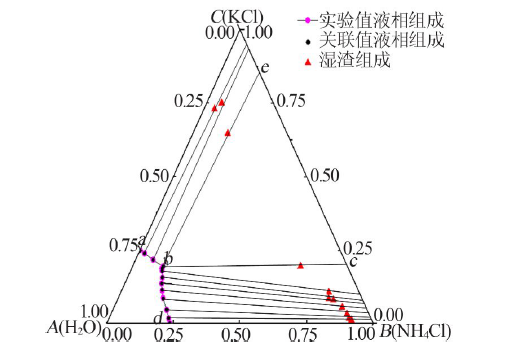
| 1,a | 23.69 | 0.00 | 23.67 | 0.00 | — | — | KCl |
| 2 | 22.46 | 1.67 | 22.36 | 1.77 | 91.19 | 1.17 | (K,NH4)Cl |
| 3 | 20.28 | 4.54 | 20.19 | 4.63 | 90.09 | 1.72 | (K,NH4)Cl |
| 4 | 16.81 | 8.65 | 17.04 | 8.42 | 88.56 | 3.18 | (K,NH4)Cl |
| 5 | 15.32 | 11.13 | 15.05 | 11.40 | 85.59 | 5.52 | (K,NH4)Cl |
| 6 | 13.90 | 13.48 | 13.81 | 13.57 | 81.03 | 7.94 | (K,NH4)Cl |
| 7 | 13.12 | 15.56 | 13.07 | 15.61 | 79.08 | 8.36 | (K,NH4)Cl |
| 8 | 11.77 | 17.96 | 11.95 | 17.78 | 78.03 | 10.63 | (K,NH4)Cl |
| 9,b | 11.54 | 18.63 | 11.42 | 18.75 | 63.01 | 19.44 | (K,NH4)Cl+(NH4,K)Cl |
| 10 | 11.48 | 19.29 | 11.48 | 19.29 | 13.10 | 64.69 | (NH4,K)Cl |
| 11 | 6.65 | 21.59 | 6.61 | 21.63 | 5.63 | 75.04 | (NH4,K)Cl |
| 12 | 2.33 | 23.80 | 2.38 | 23.75 | 3.97 | 72.98 | (NH4,K)Cl |
| 13,d | 0.00 | 24.68 | 0.00 | 24.67 | — | — | NH4Cl |
"
| 序号 | 液相组成/% | 湿固相/% | 平衡固相 | ||||
|---|---|---|---|---|---|---|---|
| 实验值 | Pitzer计算值 | ||||||
| w(NH4H2PO4) | w(KH2PO4) | w(NH4H2PO4) | w(KH2PO4) | w(NH4H2PO4) | w(KH2PO4) | ||
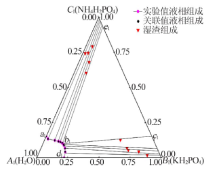
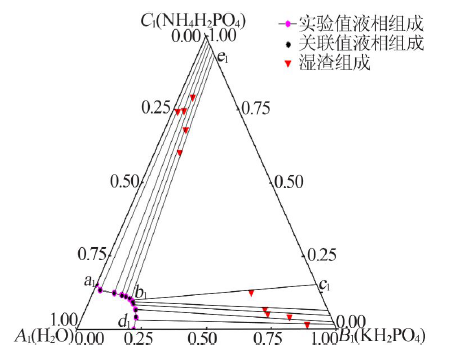
| 1,a1 | 22.01 | 0.00 | 22.02 | 0.00 | — | — | NH4H2PO4 |
| 2 | 20.92 | 4.07 | 20.68 | 4.31 | 87.93 | 1.68 | (NH4,K)H2PO4 |
| 3 | 19.28 | 6.80 | 19.51 | 6.57 | 79.82 | 4.25 | (NH4,K)H2PO4 |
| 4 | 17.37 | 8.89 | 17.39 | 8.87 | 70.91 | 5.40 | (NH4,K)H2PO4 |
| 5 | 16.68 | 9.66 | 16.97 | 9.37 | 69.08 | 6.88 | (NH4,K)H2PO4 |
| 6,b1 | 15.32 | 10.43 | 15.11 | 10.64 | 60.98 | 12.65 | (NH4,K)H2PO4+(K,NH4)H2PO4 |
| 7 | 15.33 | 10.44 | 15.16 | 10.61 | 9.75 | 60.34 | (K,NH4)H2PO4 |
| 8 | 13.35 | 11.24 | 13.43 | 11.16 | 8.16 | 68.12 | (K,NH4)H2PO4 |
| 9 | 11.50 | 11.78 | 11.74 | 11.54 | 5.31 | 79.25 | (K,NH4)H2PO4 |
| 10 | 8.34 | 12.41 | 8.31 | 12.44 | 4.22 | 74.62 | (K,NH4)H2PO4 |
| 11 | 2.36 | 13.47 | 2.37 | 13.46 | 1.91 | 74.31 | (K,NH4)H2PO4 |
| 12,d1 | 0.00 | 15.47 | 0.00 | 15.46 | — | — | KH2PO4 |
"
| 序号 | 固溶体中NH4Cl的物质的量 分数/% | 液相组成 | 活度积 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 实验值/(mol·kg-1) | Pitzer模型计算值/(mol·kg-1) | 相对误差/% | 实验值 | Pitzer模型计算值 | 相对 误差/% | |||||
| m | m | m | m | m | m | |||||
| 1,a | 0 | 4.164 2 | 4.164 2 | 4.164 2 | 4.164 2 | 0 | 0 | 7.708 6 | 7.708 6 | 0 |
| 2 | 1.756 8 | 3.970 9 | 4.382 4 | 3.953 1 | 4.364 6 | 0.449 9 | 0.407 7 | 6.732 0 | 6.970 3 | 3.540 1 |
| 3 | 2.591 9 | 3.618 4 | 4.747 4 | 3.603 2 | 4.732 2 | 0.419 7 | 0.319 9 | 6.723 0 | 7.086 9 | 5.413 0 |
| 4 | 4.766 0 | 3.025 0 | 5.194 5 | 3.065 7 | 5.235 1 | 1.343 0 | 0.782 1 | 6.545 4 | 6.804 7 | 3.960 4 |
| 5 | 8.247 3 | 2.794 0 | 5.623 1 | 2.745 5 | 5.574 5 | 1.737 1 | 0.863 2 | 6.597 9 | 6.580 2 | 0.268 3 |
| 6 | 12.015 8 | 2.567 5 | 6.037 8 | 2.550 5 | 6.020 8 | 0.662 0 | 0.281 5 | 7.354 8 | 6.929 1 | 5.788 1 |
| 7 | 12.841 7 | 2.467 6 | 6.546 3 | 2.457 7 | 6.536 4 | 0.402 9 | 0.151 9 | 7.673 8 | 7.751 4 | 1.012 0 |
| 8 | 15.956 9 | 2.246 8 | 7.0250 | 2.281 4 | 7.059 5 | 1.539 1 | 0.492 2 | 8.363 5 | 8.277 0 | 1.034 0 |
| 9,b | 30.069 6 | 2.216 7 | 7.204 4 | 2.193 7 | 7.181 4 | 1.037 9 | 0.319 4 | 9.010 8 | 8.519 5 | 5.452 4 |
| 10 | 87.313 5 | 2.224 3 | 7.433 5 | 2.225 2 | 7.410 3 | 0.038 3 | 0.312 3 | 10.621 0 | 10.444 0 | 1.666 1 |
| 11 | 94.891 8 | 1.243 1 | 6.867 7 | 1.236 0 | 6.860 7 | 0.569 9 | 0.103 2 | 8.675 9 | 9.054 9 | 4.368 4 |
| 12 | 96.243 5 | 0.423 1 | 6.446 4 | 0.432 5 | 6.455 8 | 3.096 9 | 0.202 9 | 8.206 2 | 8.089 8 | 1.418 8 |
| 13,d | 100 | 0 | 6.225 1 | 0 | 6.225 1 | 0 | 0 | 14.154 0 | 14.154 0 | 0 |
"
| 序号 | 固溶体中NH4Cl的物质的量 分数/% | 液相组成 | 活度积 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 实验值/(mol·kg-1) | Pitzer模型计算值/(mol·kg-1) | 相对误差/% | 实验值 | Pitzer模型计算值 | 相对 误差/% | |||||
| m | m | m | m | m | m | |||||
| 1,a1 | 100 | 0 | 2.453 4 | 0 | 2.453 4 | 0 | 0 | 0.3864 | 0.386 4 | 0 |
| 2 | 86.082 9 | 0.398 7 | 2.823 3 | 0.422 4 | 2.846 9 | 5.935 6 | 0.838 2 | 0.322 8 | 0.325 0 | 0.678 2 |
| 3 | 77.334 2 | 0.676 0 | 2.943 4 | 0.652 9 | 2.920 4 | 3.405 8 | 0.782 1 | 0.299 8 | 0.300 3 | 0.176 6 |
| 4 | 70.160 4 | 0.885 9 | 2.933 7 | 0.883 8 | 2.931 6 | 0.231 0 | 0.069 8 | 0.287 3 | 0.287 7 | 0.125 0 |
| 5 | 67.510 1 | 0.963 6 | 2.932 2 | 0.935 1 | 2.903 7 | 2.965 4 | 0.974 6 | 0.281 5 | 0.281 9 | 0.165 8 |
| 6,b1 | 63.867 2 | 1.032 2 | 2.825 9 | 1.052 6 | 2.846 3 | 1.972 2 | 0.720 4 | 0.272 7 | 0.274 4 | 0.616 6 |
| 7 | 63.860 2 | 1.033 5 | 2.828 8 | 1.050 0 | 2.845 4 | 1.604 4 | 0.586 2 | 0.273 0 | 0.274 3 | 0.485 5 |
| 8 | 58.835 5 | 1.095 2 | 2.634 3 | 1.087 8 | 2.626 8 | 0.684 1 | 0.284 4 | 0.232 4 | 0.238 9 | 2.783 3 |
| 9 | 54.018 2 | 1.128 3 | 2.431 4 | 1.104 8 | 2.407 9 | 2.077 3 | 0.964 0 | 0.223 7 | 0.221 9 | 0.776 9 |
| 10 | 44.712 2 | 1.150 7 | 2.065 5 | 1.153 9 | 2.068 7 | 0.278 2 | 0.155 0 | 0.198 2 | 0.198 3 | 0.032 8 |
| 11 | 17.412 5 | 1.175 9 | 1.419 7 | 1.174 7 | 1.418 5 | 0.103 0 | 0.085 3 | 0.176 2 | 0.176 2 | 0.006 2 |
| 12 | 0 | 1.344 8 | 1.344 8 | 1.344 8 | 1.344 8 | 0 | 0 | 0.256 6 | 0.256 6 | 0 |
| 13,d1 | 100 | 0 | 2.453 4 | 0 | 2.453 4 | 0 | 0 | 0.386 4 | 0.386 4 | 0 |
| [1] |
Pitzer K S. Thermodynamics of electrolytes.I.Theoretical basis and general equations[J]. The Journal of Physical Chemistry, 1973,77(2):268-277.
doi: 10.1021/j100621a026 |
| [2] |
Pitzer K S, Mayorga G. Thermodynamics of electrolytes.Ⅲ.Activity and osmotic coefficients for 2-2 electrolytes[J]. Journal of Solution Chemistry, 1974,3(7):539-546.
doi: 10.1007/BF00648138 |
| [3] |
Wilson G M. Vapor-Liquid equilibrium.XI.A new expression for the excess free energy of mixing[J]. Journal of the American Chemical Society, 1964,86(2):127-130.
doi: 10.1021/ja01056a002 |
| [4] | Renon H, Prausnitz J M. Estimation of parameters for the NRTL eq-uation for excess gibbs energies of strongly nonideal liquid mixtur-es[J]. Industrial & Engineering Chemistry Process Design and De-velopment, 1969,8(3):413-419. |
| [5] | Fu Y H, Sandier S I, Orbey H. A modified UNIQUAC model that in-cludes hydrogen bonding[J]. Industrial & Engineering Chemistry Research, 1995,34(12):4351-4363. |
| [6] | Fatima F, Cristina F, António A. Solubilities for six ternary systems:NaCl+NH4Cl+H2O,KCl+NH4Cl+H2O,NaCl+LiCl+H2O,KCl+LiCl+H2O,NaCl+AlCl3+H2O,and KCl+AlCl3+H2O at T=(298 to 333) K[J]. Journal of Chemical & Engineering Data, 2005,50(4):1470-1477. |
| [7] | Yu X, Zeng Y, Zhang Z. Solid-liquid metastable phase equilibria in the ternary systems KCl+NH4Cl+H2O and NH4Cl+MgCl2+H2O at 298.15 K[J]. Journal of Chemical & Engineering Data, 2012,57(6):1759-1765. |
| [8] | Zhao B, Geng G Y, Chen J X, et al. The ternary system phase equili-brium of KCl-NH4Cl-H2O at 80 ℃[J]. Advanced Materials Resear-ch, 2013,834/835/836:519-522. |
| [9] | 赵长伟, 马沛生, 郭瓦力, 等. KCl-NH4Cl-H2O三元水盐体系溶解度的研究[J]. 化学工业与工程, 2003,20(3):145-149. |
| [10] | 樊彩梅, 舒兰, 余华瑞, 等. 含固溶体三元体系K2SO4-(NH4)2SO4-H2O 和 KCl-NH4Cl-H2O溶解度的计算[J]. 高校化学工程学报, 1998,12(1):65-70. |
| [11] | 胡雪, 朱静, 王睿哲, 等.( NH2)2CO-NH4H2PO4-H2O三元系10 ℃相平衡研究[J]. 无机盐工业, 2019,51(5):47-50. |
| [12] | 吴强, 胡雪, 朱静, 等. KH2PO4-KCl-H2O、NH4H2PO4-NH4Cl-H2O三元体系283.15 K相平衡研究[J]. 无机盐工业, 2020,52(11):24-28. |
| [13] | 刘光启, 马连湘, 刘杰. 化学化工物性数据手册无机卷[M]. 北京: 化学工业出版社, 2004. |
| [14] | 陆杏英. 钼锑抗比色法测定土壤中的总磷[J]. 环境科学技术, 1991,4(3):33-34. |
| [15] | 宋学兰, 巫倩, 李艳. 硝酸中微量氯离子测定方法探讨[J]. 中氮肥, 2009(1):59-63. |
| [16] |
刘越, 李琰, 陈珮豪, 等. 甲醛法测定铵盐中氮含量实验的改进[J]. 大学化学, 2015,30(1):45-48.
doi: 10.3866/PKU.DXHX20150109 |
| [17] | Harvie C E, Eugster H P, Weare J H. Mineral equilibria in the six-component seawater system,Na-K-Mg-Ca-SO4-Cl-H2O at 25 ℃.Ⅱ:Compositions of the saturated solutions[J]. Geochimica Et Co-smochimica Acta, 1982,46(9):1603-1618. |
| [18] | Pitzer K S, Mayorga G. Thermodynamics of electrolytes.Ⅱ.Activity and osmotic coefficients for strong electrolytes with one or both ions univalent[J]. The Journal of Physical Chemistry, 1973,77(19):2300-2308. |
| [19] | Pitzer K S, Kim J J. Thermodynamics of electrolytes.Ⅳ.Activity and osmotic coefficients for mixed electrolytes[J]. Journal of the Ame-rican Chemical Society, 1974,96(18):5701-5707. |
| [20] | Partanen J I. Mean activity coefficients and osmotic coefficients in aqueous solutions of salts of ammonium ions with univalent anions at 25 ℃[J]. Journal of Chemical & Engineering Data, 2012,57(10):2654-2666. |
| [21] |
Silvester L F, Pitzer K S. Thermodynamics of electrolytes.X.Enth-alpy and the effect of temperature on the activity coefficients[J]. Journal of Solution Chemistry, 1978,7(5):327-337.
doi: 10.1007/BF00662893 |
| [1] | Liu Juexin,Zheng Chenggang,Ye Shichao. Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal [J]. Inorganic Chemicals Industry, 2021, 53(1): 62-64. |
| [2] | Zhang Liyuan,Wang Gang,Qi Meiling,Xie Yulong. Effect of surfactants on metastable zone and induction period of KCl crystals in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(6): 46-49. |
| [3] | Wang Jukui,Dong Xingfeng,Zhao Dong,Wang Shiqiang,Guo Yafei,Deng Tianlong. Solid-liquid phase equilibria in quaternary system lithium borate-potassium borate-magnesium borate-water at 308.15 K [J]. Inorganic Chemicals Industry, 2020, 52(5): 27-30. |
| [4] | Qi Meiling,Wang Gang,Zhang Liyuan,Xie Yulong. Determination of KCl solubility,interfacial region and induction period in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(5): 45-49. |
| [5] | Wang Yubin,Wang Wangbo,Zhu Xinfeng,Xun Jingwen,Dang Weiben. Effect of different magnesium salts on solubility behavior of calcium carbonate under magnetization [J]. Inorganic Chemicals Industry, 2020, 52(3): 35-38. |
| [6] | Wu Qiang,Hu Xue,Zhu Jing,Yang Jiamin,Li Tianxiang. Phase equilibrium of KH2PO4-KCl-H2O and NH4H2PO4-NH4Cl-H2O ternary system at 283.15 K [J]. Inorganic Chemicals Industry, 2020, 52(11): 24-28. |
| [7] | Cao Peng. Effect of acid soluble main reverse on solubility of solid phase of titanium white production by sulfuric acid process [J]. Inorganic Chemicals Industry, 2019, 51(9): 54-56. |
| [8] | Liu Leilei,Xia Haijian,Feng Weiliang,Wang Tielin,Wang Cunwen,Wang Weiguo,Jiang Zhensheng. Determination and correlation of solubility of potassium fluosilicate in dilute sulfuric acid solution [J]. Inorganic Chemicals Industry, 2019, 51(8): 17-19. |
| [9] | Zhang Wenhao,Yang Libin,Sha Zuoliang,Wang Yanfei,Zhu Liang,Zhao Xiaoyu. Determination of crystalline metastable zone and primary nucleationanalysis of lanthanum chloride heptahydrate [J]. Inorganic Chemicals Industry, 2019, 51(7): 43-47. |
| [10] | Wu Linxin,Zeng Ying,Chen Peijun,Huang Peng,Chen Yu,Sun Jiu,Yu Xudong. Stable phase equilibrium of the ternary system K +,Cs +//SO4 2--H2O at 298.2 K [J]. Inorganic Chemicals Industry, 2019, 51(6): 17-20. |
| [11] | Hu Xue,Zhu Jing,Wang Ruizhe,Li Hongguo,Li Tianxiang. Research on phase equilibrium of ternary system of (NH2)2CO-NH4H2PO4-H2O at 10 ℃ [J]. Inorganic Chemicals Industry, 2019, 51(5): 41-44. |
| [12] | Zhang Chan,Cheng Wenting,Cheng Huaigang,Guo Yanxia,Cheng Fangqin. Simulation and determination of AlCl3·6H2O solubility in FeCl3-CaCl2-HCl-H2O system [J]. Inorganic Chemicals Industry, 2019, 51(5): 61-65. |
| [13] | Chen Lifang,Zhang Qinqin,Lin Tian,Yue Peiying. Determination and correlation of solubility of boric acid in calcium chloride solution [J]. Inorganic Chemicals Industry, 2019, 51(5): 70-73. |
| [14] | REN Xiao-Jing, HUANG Xue-Li. Phase equilibria in the quatemary system of Li+,Mg2+∥Cl-,SO42--H2O at 273.15 K [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 13-. |
| [15] | YANG Fan, JIE Tian, LIU Fei. Measurement of the solubility of feed-grade urea phosphate in water by laser monitoring technique [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(4): 69-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|