Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (1): 62-64.doi: 10.11962/1006-4990.2020-0082
Previous Articles Next Articles
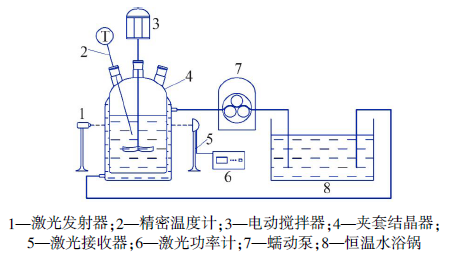
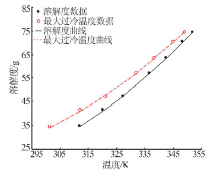
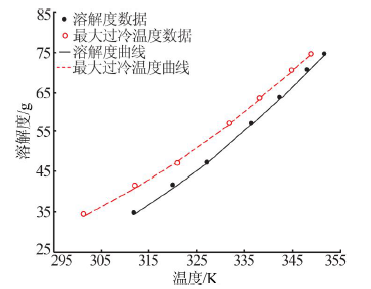
Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal
Liu Juexin( ),Zheng Chenggang,Ye Shichao(
),Zheng Chenggang,Ye Shichao( )
)
- School of Chemical Engineering,Sichuan University,Chengdu 610065,China
-
Received:2020-07-27Online:2021-01-10Published:2021-01-08 -
Contact:Ye Shichao E-mail:juexinliu@sina.com;shichaoye@sina.com
CLC Number:
Cite this article
Liu Juexin,Zheng Chenggang,Ye Shichao. Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal[J]. Inorganic Chemicals Industry, 2021, 53(1): 62-64.
share this article
| [1] | 胡秀英, 党亚固, 郑纯智, 等. 磷酸二氢钾结晶过程研究[J]. 化工矿物与加工, 2009,38(1):9-12. |
| [2] | 汤秀华, 李军, 周堃, 等. 磷酸二氢钾结晶介稳区宽度的研究[J]. 无机盐工业, 2007,39(7):27-29. |
| [3] | 周存, 孙菲. 水溶液中磷酸二氢钾结晶影响因素的研究[J]. 化工新型材料, 2017,45(9):193-195. |
| [4] | 黄美英, 蒋德敏, 钟本和, 等. 磷酸二氢钾结晶工艺控制研究[J]. 化工矿物与加工, 2018,47(12):13-18,32. |
| [5] | 保英莲, 张志强, 李小松, 等. 磷酸二氢钾结晶过程特性研究[J]. 无机盐工业, 2016,48(11):29-34. |
| [6] | 韩佳宾, 王静康. 咖啡因在水和乙醇中介稳区的测定[J]. 天津大学学报, 2003(6):765-768. |
| [7] | 何兴学, 孙勤, 杨阿三, 等. 醋酸钠介稳区的测定[J]. 化学工程, 2012,40(7):43-45. |
| [8] | 胡秀英, 马迪, 汪斌, 等. 铁离子对磷酸二氢钾结晶过程的影响[J]. 无机盐工业, 2013,45(2):11-14. |
| [9] | 丁绪淮, 谈遒. 工业结晶[M]. 北京: 化学工业出版社, 1985. |
| [10] |
Yang J, Qian G, Wu Y, et al. Thermodynamic analysis of the solu-bility of polymorphic cytarabine in a variety of pure solvents[J]. Fluid Phase Equilibria, 2017,445:1-6.
doi: 10.1016/j.fluid.2017.04.021 |
| [11] | Apelblat A, Manzurola E. Solubilities of o-acetylsalicylic,4-amino-salicylic,3,5-dinitrosalicylic,and p-toluic acid,and magnesium-DL-aspartate in water from T=(278 to 348) K[J]. Journal of Che-mical Thermodynamics, 1999,31(1):85-91. |
| [12] | 汪朝强, 唐浩, 明大增, 等. 磷酸二氢钾生产方法现状及发展前景[J]. 无机盐工业, 2017,49(6):7-11. |
| [13] | 彭敬慧, 冯梦龙, 黄卓, 等. 磷酸二氢钾的制备方法综述[J]. 磷肥与复肥, 2018,33(10):15-17. |
| [14] | 吴宇川, 何兵兵, 薛绍秀, 等. 磷酸二氢钾的制备与应用研究进展[J]. 磷肥与复肥, 2017,32(2):30-34. |
| [15] |
Ulrich J, Strege C. Some aspects of the importance of metastable zone width and nucleation in industrial crystallizers[J]. Journal of Crystal Growth, 2002,237/239:2130-2135.
doi: 10.1016/S0022-0248(01)02284-9 |
| [1] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| [2] | Zhang Liyuan,Wang Gang,Qi Meiling,Xie Yulong. Effect of surfactants on metastable zone and induction period of KCl crystals in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(6): 46-49. |
| [3] | Wang Jukui,Dong Xingfeng,Zhao Dong,Wang Shiqiang,Guo Yafei,Deng Tianlong. Solid-liquid phase equilibria in quaternary system lithium borate-potassium borate-magnesium borate-water at 308.15 K [J]. Inorganic Chemicals Industry, 2020, 52(5): 27-30. |
| [4] | Qi Meiling,Wang Gang,Zhang Liyuan,Xie Yulong. Determination of KCl solubility,interfacial region and induction period in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(5): 45-49. |
| [5] | Wang Yubin,Wang Wangbo,Zhu Xinfeng,Xun Jingwen,Dang Weiben. Effect of different magnesium salts on solubility behavior of calcium carbonate under magnetization [J]. Inorganic Chemicals Industry, 2020, 52(3): 35-38. |
| [6] | Wu Qiang,Hu Xue,Zhu Jing,Yang Jiamin,Li Tianxiang. Phase equilibrium of KH2PO4-KCl-H2O and NH4H2PO4-NH4Cl-H2O ternary system at 283.15 K [J]. Inorganic Chemicals Industry, 2020, 52(11): 24-28. |
| [7] | Cao Peng. Effect of acid soluble main reverse on solubility of solid phase of titanium white production by sulfuric acid process [J]. Inorganic Chemicals Industry, 2019, 51(9): 54-56. |
| [8] | Liu Leilei,Xia Haijian,Feng Weiliang,Wang Tielin,Wang Cunwen,Wang Weiguo,Jiang Zhensheng. Determination and correlation of solubility of potassium fluosilicate in dilute sulfuric acid solution [J]. Inorganic Chemicals Industry, 2019, 51(8): 17-19. |
| [9] | Zhang Wenhao,Yang Libin,Sha Zuoliang,Wang Yanfei,Zhu Liang,Zhao Xiaoyu. Determination of crystalline metastable zone and primary nucleationanalysis of lanthanum chloride heptahydrate [J]. Inorganic Chemicals Industry, 2019, 51(7): 43-47. |
| [10] | Zhang Chan,Cheng Wenting,Cheng Huaigang,Guo Yanxia,Cheng Fangqin. Simulation and determination of AlCl3·6H2O solubility in FeCl3-CaCl2-HCl-H2O system [J]. Inorganic Chemicals Industry, 2019, 51(5): 61-65. |
| [11] | Chen Lifang,Zhang Qinqin,Lin Tian,Yue Peiying. Determination and correlation of solubility of boric acid in calcium chloride solution [J]. Inorganic Chemicals Industry, 2019, 51(5): 70-73. |
| [12] | Zhou Xiumei,Sun Guixing. Optimization technology for stable operation of ammonium chloride crystal system in potassium dihydrogen phosphate production plant [J]. Inorganic Chemicals Industry, 2019, 51(12): 58-60. |
| [13] | REN Xiao-Jing, HUANG Xue-Li. Phase equilibria in the quatemary system of Li+,Mg2+∥Cl-,SO42--H2O at 273.15 K [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 13-. |
| [14] | YANG Fan, JIE Tian, LIU Fei. Measurement of the solubility of feed-grade urea phosphate in water by laser monitoring technique [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(4): 69-. |
| [15] | BAO Ying-Lian, ZHANG Zhi-Qiang, LI Xiao-Song, KUANG Tian-Liang. Study on characteristics of crystallization process of potassium dihydrogen phosphate [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(11): 29-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|