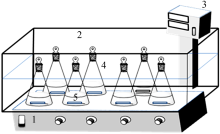
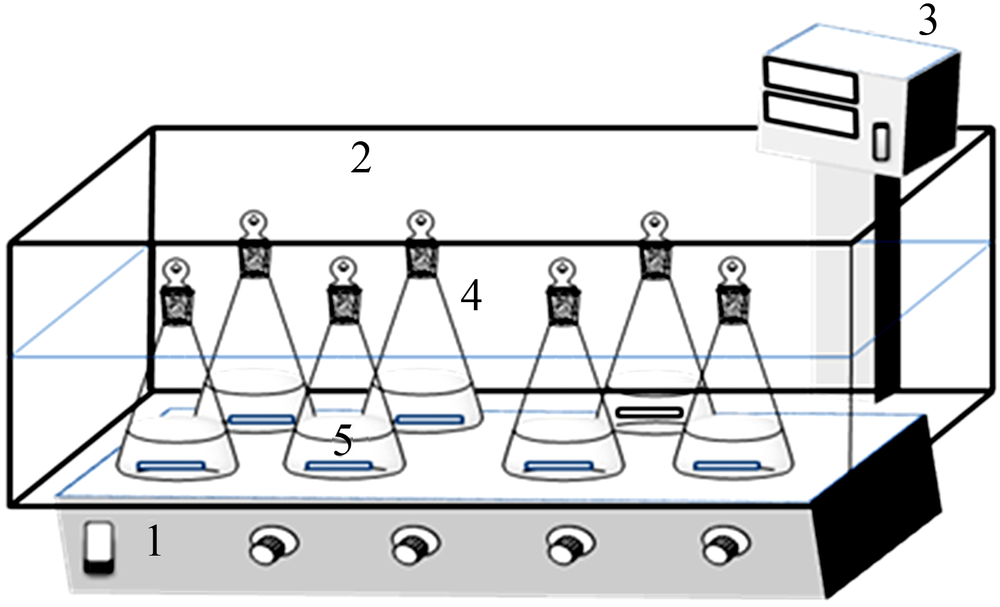
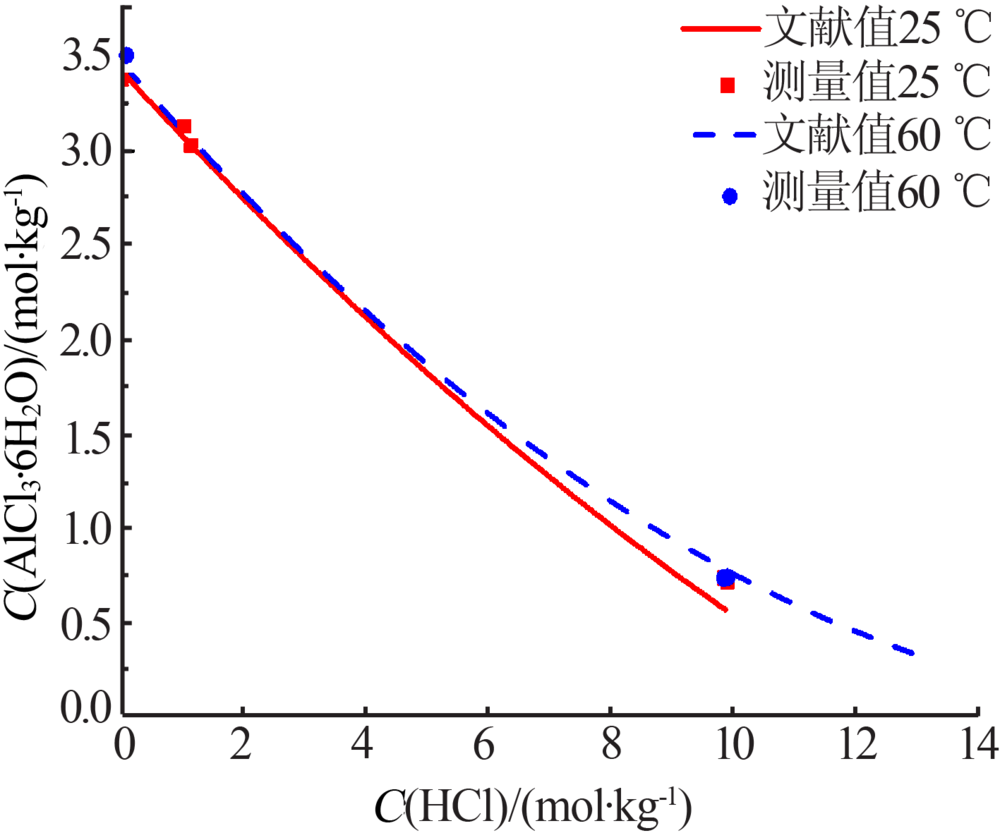
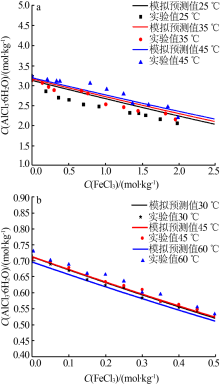
Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (5): 61-65.
• Environment·Health·Safety • Previous Articles Next Articles
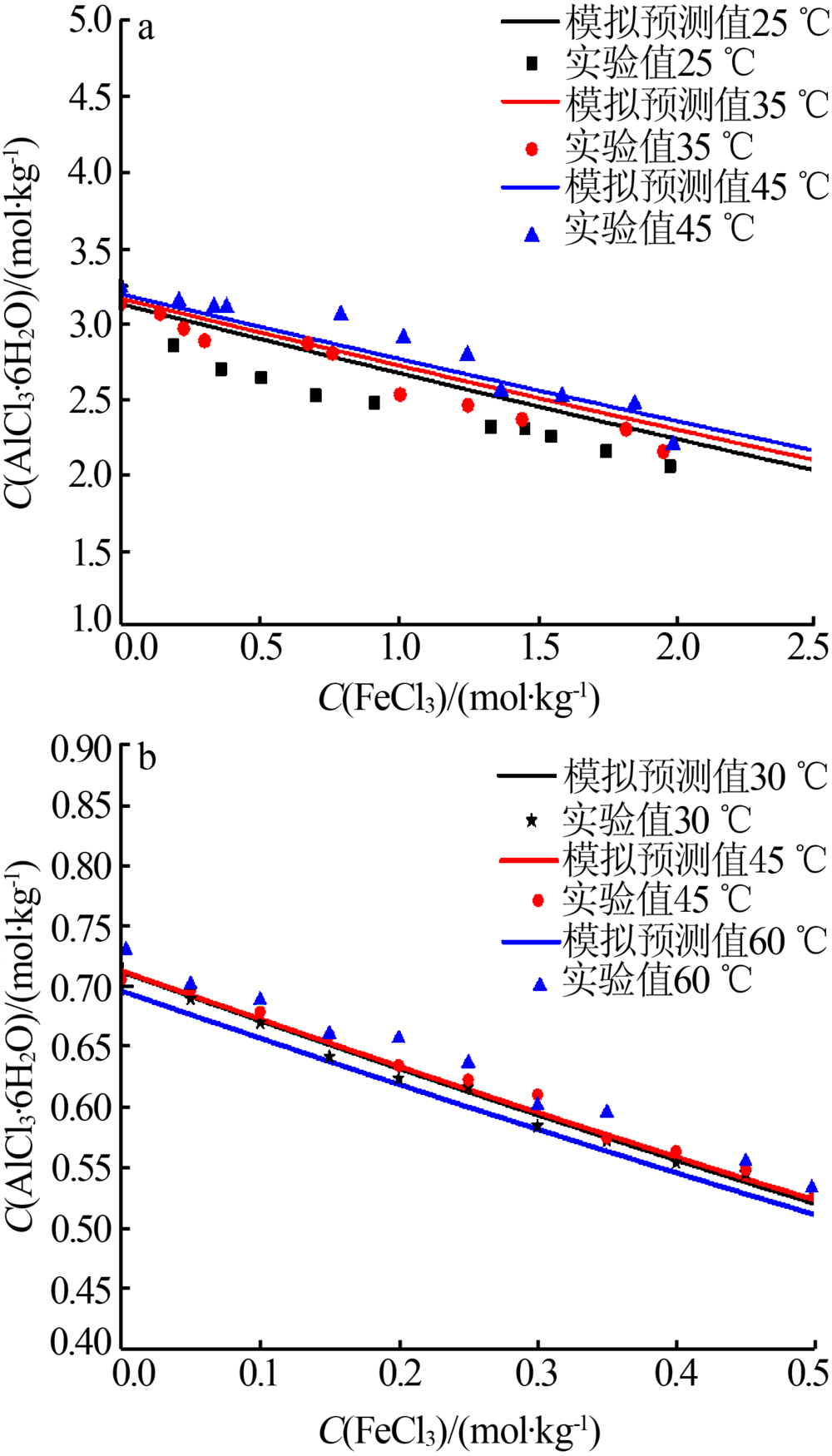
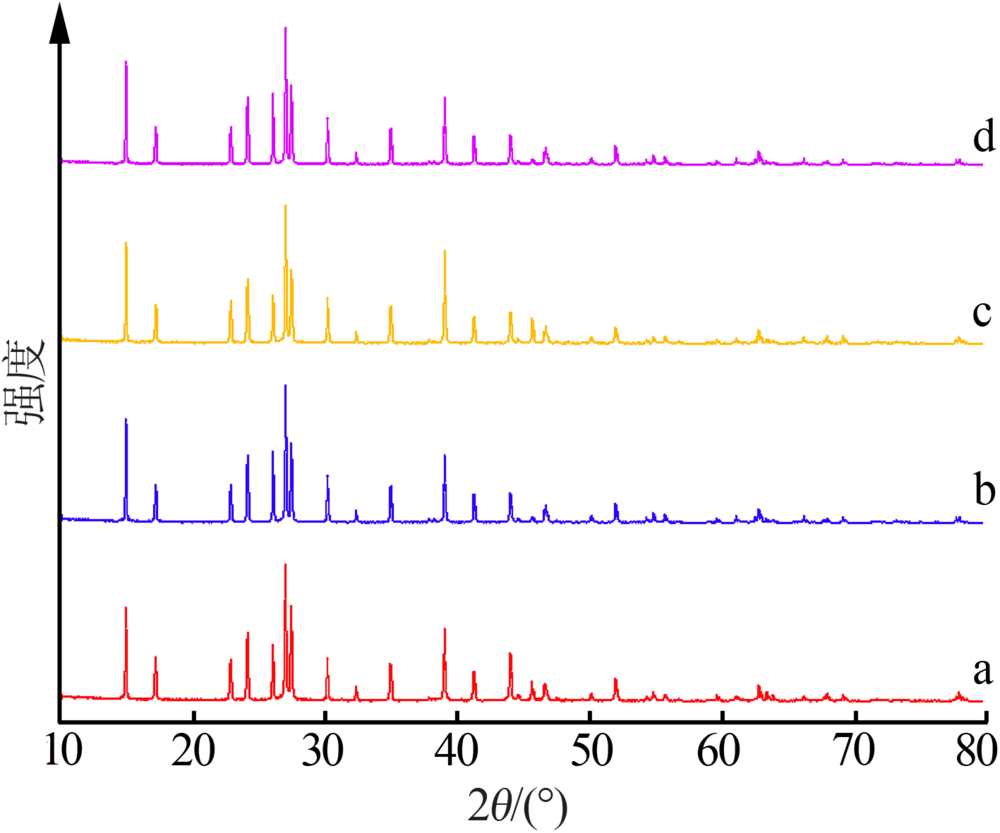
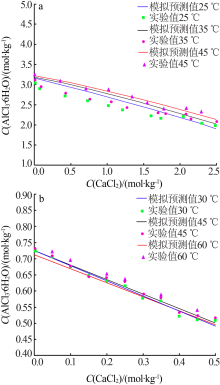
Simulation and determination of AlCl3·6H2O solubility in FeCl3-CaCl2-HCl-H2O system
Zhang Chan,Cheng Wenting( ),Cheng Huaigang,Guo Yanxia,Cheng Fangqin
),Cheng Huaigang,Guo Yanxia,Cheng Fangqin
- Institute of Resources and Environmental Engineering,State Environmental Protection Key Laboratory on Efficient Resource-utilization Techniques of Coal Waste,Shanxi University,Taiyuan 030006,China
-
Received:2018-11-12Online:2019-05-10Published:2020-04-28 -
Contact:Cheng Wenting E-mail:wtcheng@sxu.edu.cn
CLC Number:
Cite this article
Zhang Chan,Cheng Wenting,Cheng Huaigang,Guo Yanxia,Cheng Fangqin. Simulation and determination of AlCl3·6H2O solubility in FeCl3-CaCl2-HCl-H2O system[J]. Inorganic Chemicals Industry, 2019, 51(5): 61-65.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] |
Takaara T, Sano D, Masago Y , et al. Surface-retained organic matterof Microcystisaeruginosa inhibiting coagulation with polyaluminum chloride in drinking water treatment[J]. Water Research, 2010,44(13):3781-3786.
doi: 10.1016/j.watres.2010.04.030 pmid: 20570314 |
| [2] | 潘碌亭, 束玉保, 王键 , 等. 聚合氯化铝絮凝剂的制备技术研究现状与进展[J]. 工业用水与废水, 2008,39(3):21-25. |
| [3] | 郑怀礼, 陆兰英, 范伟 , 等. 聚合氯化铝的制备及在微污染水处理中的应用[J]. 化学研究与应用, 2012,24(4):626-629. |
| [4] | 孙天旭, 王立 . 三氯化铝系列催化剂在Friedel-Crafts 酰基化反应中的应用进展[J]. 精细石油化工, 2006,23(1):57-62. |
| [5] | Park H C, Park Y J, Stevens R . Synjournal of alumina from high purity alum derived from coal fly ash[J]. Materials Science & Engineering A, 2004,367(1/2):166-170. |
| [6] |
Matjie R H, Bunt J R, van Heerden J H P . Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal[J]. Minerals Engineering, 2005,18(3):299-310.
doi: 10.1016/j.mineng.2004.06.013 |
| [7] | Eisele J A, Bauer D J, Shanks D E . Bench-scale studies to recover alumina from clay by a hydrochloric acid process[J]. Industrial & Engineering Chemistry Product Research & Development, 1983,22(1):105-110. |
| [8] | Gao W C, Li Z B . A practical approach to produce Mg-Al spinel based on the modeling of phase equilibria for NH4Cl-MgCl2-AlCl3-H2O system[J]. AIChE Journal, 2013,59(6):1855-1867. |
| [9] | Yuan M X, Qiao X C, Yu J G . Phase equilibria of AlCl3+FeCl3+H2O,AlCl3+CaCl2+H2O,and FeCl3+CaCl2+H2O at 298.15 K[J]. Journal of Chemical & Engineering Data, 2016,61(5):1749-1755. |
| [10] | Wang Junfeng, Camille Petit, Xiangping Zhang , et al. Phase equilibrium study of the AlCl3-CaCl2-H2O system for the production of aluminum chloride hexahydrate from Ca-rich flue ash[J]. Journal of Chemical & Engineering Data, 2016,61(1):359-369. |
| [11] | Fátima Farelo, Cristina Fernandes, António Avelino . Solubilities for six ternary systems:NaCl+NH4Cl+H2O,KCl+NH4Cl+H2O,NaCl+LiCl+H2O,KCl+LiCl+H2O,NaCl+AlCl3+H2O,and KCl+AlCl3+H2O at T=(298 to 333) K[J]. Cheminform, 2010,36(40):1470-1477. |
| [12] | Brown R R, Daut G E, Mrazek R V , et al. Solubility and activity of aluminum chloride in aqueous hydrochloric acid solutions[R]. Albany,OR:Bureau of Mines , 1979. |
| [13] | André L, Christov C, Lassin A, et al. Pitzer ion-interaction parameters for Al(Ⅲ) in the {H+Na+K+Ca+Mg+Cl+H2O} system up to salts solubility at 298.15 K[C]// Heidelberg:ABC-Salt Ⅳ Workshop 2015,2015. |
| [14] | 胡程耀, 黄培 . 固体溶解度测定方法的近期研究进展[J]. 药物分析杂志, 2010,22(4):761-766. |
| [15] | 付梦源, 程文婷, 程芳琴 . AlCl3·6H2O在Fe(Ⅲ)-K-Ca-Cl-H2O体系中溶解度的测定[J]. 中国科技论文, 2015(24):2856-2862. |
| [16] | 崔慧霞, 程文婷, 郭彦霞 , 等. 六水合氯化铝在不同氯化物体系中的溶解度现象研究[J]. 无机盐工业, 2013,45(5):9-12. |
| [1] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| [2] | Liu Juexin,Zheng Chenggang,Ye Shichao. Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal [J]. Inorganic Chemicals Industry, 2021, 53(1): 62-64. |
| [3] | Zhang Liyuan,Wang Gang,Qi Meiling,Xie Yulong. Effect of surfactants on metastable zone and induction period of KCl crystals in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(6): 46-49. |
| [4] | Wang Jukui,Dong Xingfeng,Zhao Dong,Wang Shiqiang,Guo Yafei,Deng Tianlong. Solid-liquid phase equilibria in quaternary system lithium borate-potassium borate-magnesium borate-water at 308.15 K [J]. Inorganic Chemicals Industry, 2020, 52(5): 27-30. |
| [5] | Qi Meiling,Wang Gang,Zhang Liyuan,Xie Yulong. Determination of KCl solubility,interfacial region and induction period in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(5): 45-49. |
| [6] | Wang Yubin,Wang Wangbo,Zhu Xinfeng,Xun Jingwen,Dang Weiben. Effect of different magnesium salts on solubility behavior of calcium carbonate under magnetization [J]. Inorganic Chemicals Industry, 2020, 52(3): 35-38. |
| [7] | Wu Qiang,Hu Xue,Zhu Jing,Yang Jiamin,Li Tianxiang. Phase equilibrium of KH2PO4-KCl-H2O and NH4H2PO4-NH4Cl-H2O ternary system at 283.15 K [J]. Inorganic Chemicals Industry, 2020, 52(11): 24-28. |
| [8] | Cao Peng. Effect of acid soluble main reverse on solubility of solid phase of titanium white production by sulfuric acid process [J]. Inorganic Chemicals Industry, 2019, 51(9): 54-56. |
| [9] | Liu Leilei,Xia Haijian,Feng Weiliang,Wang Tielin,Wang Cunwen,Wang Weiguo,Jiang Zhensheng. Determination and correlation of solubility of potassium fluosilicate in dilute sulfuric acid solution [J]. Inorganic Chemicals Industry, 2019, 51(8): 17-19. |
| [10] | Zhang Wenhao,Yang Libin,Sha Zuoliang,Wang Yanfei,Zhu Liang,Zhao Xiaoyu. Determination of crystalline metastable zone and primary nucleationanalysis of lanthanum chloride heptahydrate [J]. Inorganic Chemicals Industry, 2019, 51(7): 43-47. |
| [11] | Chen Lifang,Zhang Qinqin,Lin Tian,Yue Peiying. Determination and correlation of solubility of boric acid in calcium chloride solution [J]. Inorganic Chemicals Industry, 2019, 51(5): 70-73. |
| [12] | REN Xiao-Jing, HUANG Xue-Li. Phase equilibria in the quatemary system of Li+,Mg2+∥Cl-,SO42--H2O at 273.15 K [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 13-. |
| [13] | YANG Fan, JIE Tian, LIU Fei. Measurement of the solubility of feed-grade urea phosphate in water by laser monitoring technique [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(4): 69-. |
| [14] | . Thermodynamic analysis on system of ZnO-NH3-NH4HCO3-H2O in process of zinc oxide leaching [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(6): 30-. |
| [15] | . Study on synthesis of easing soluble aluminum hydroxide by carbonation decomposition [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(6): 47-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|