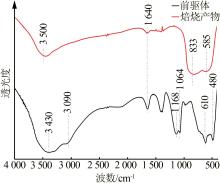
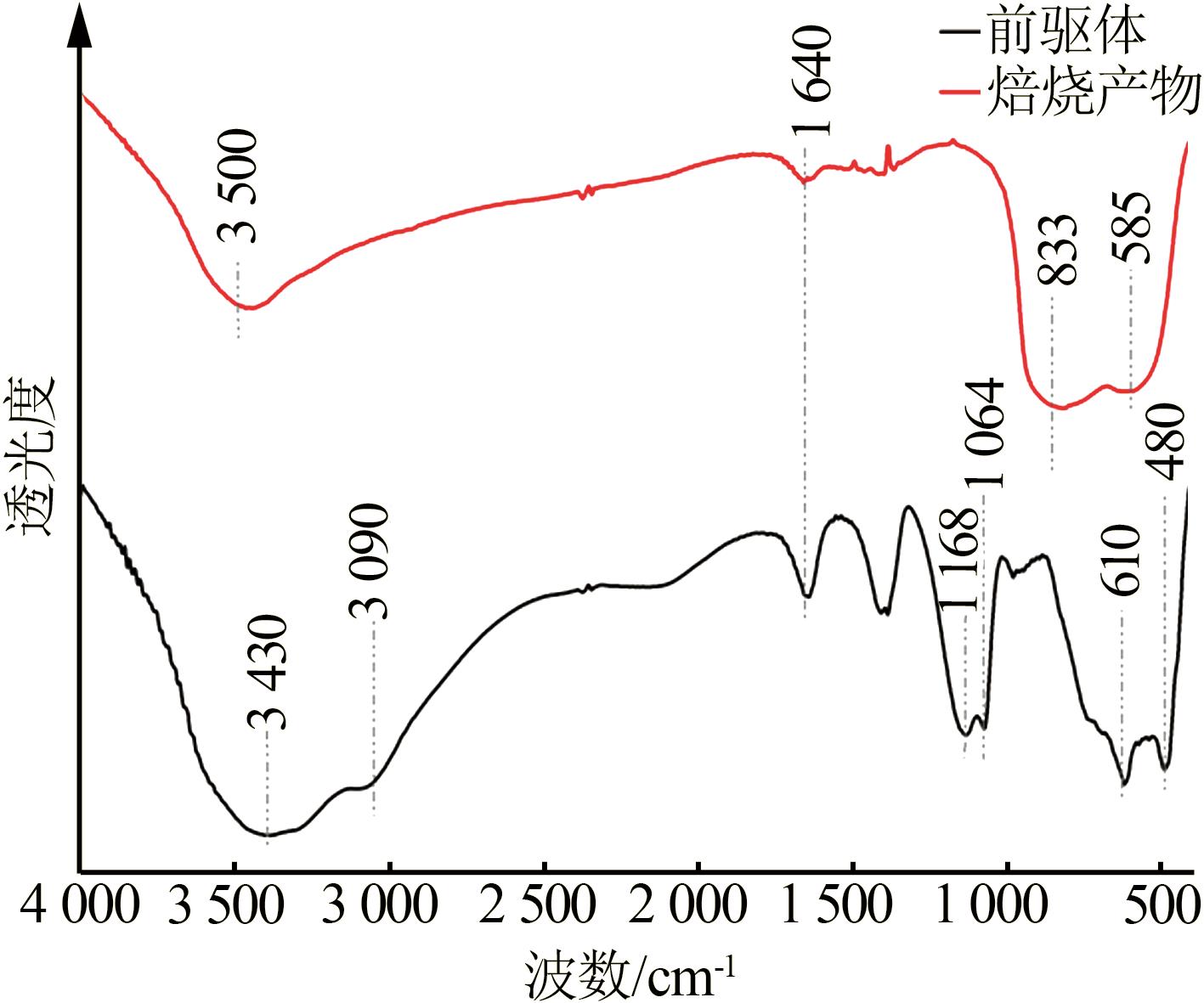
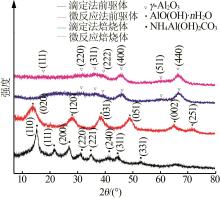
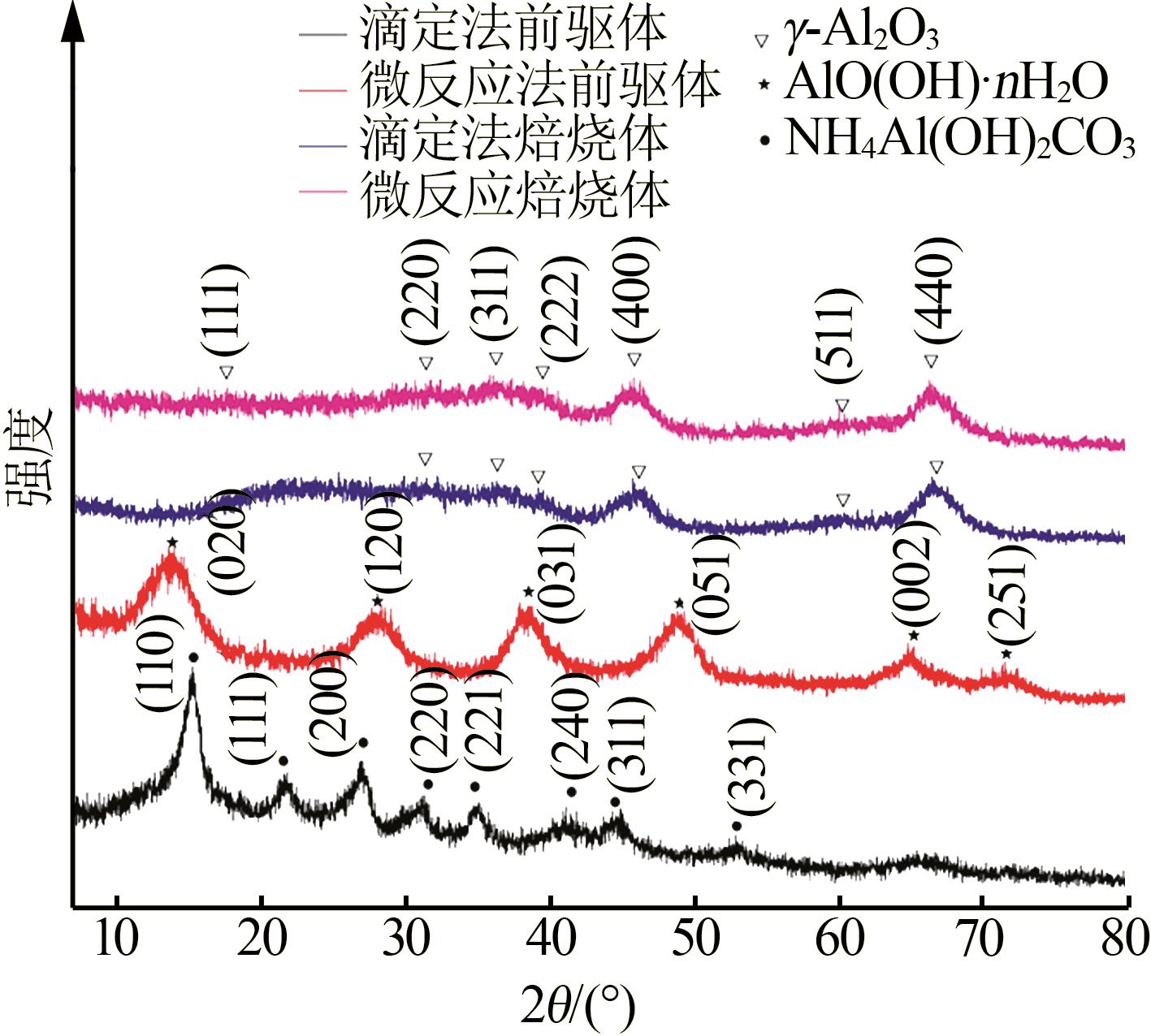
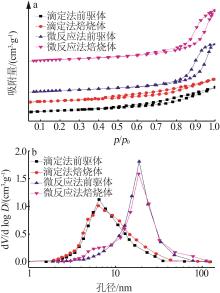
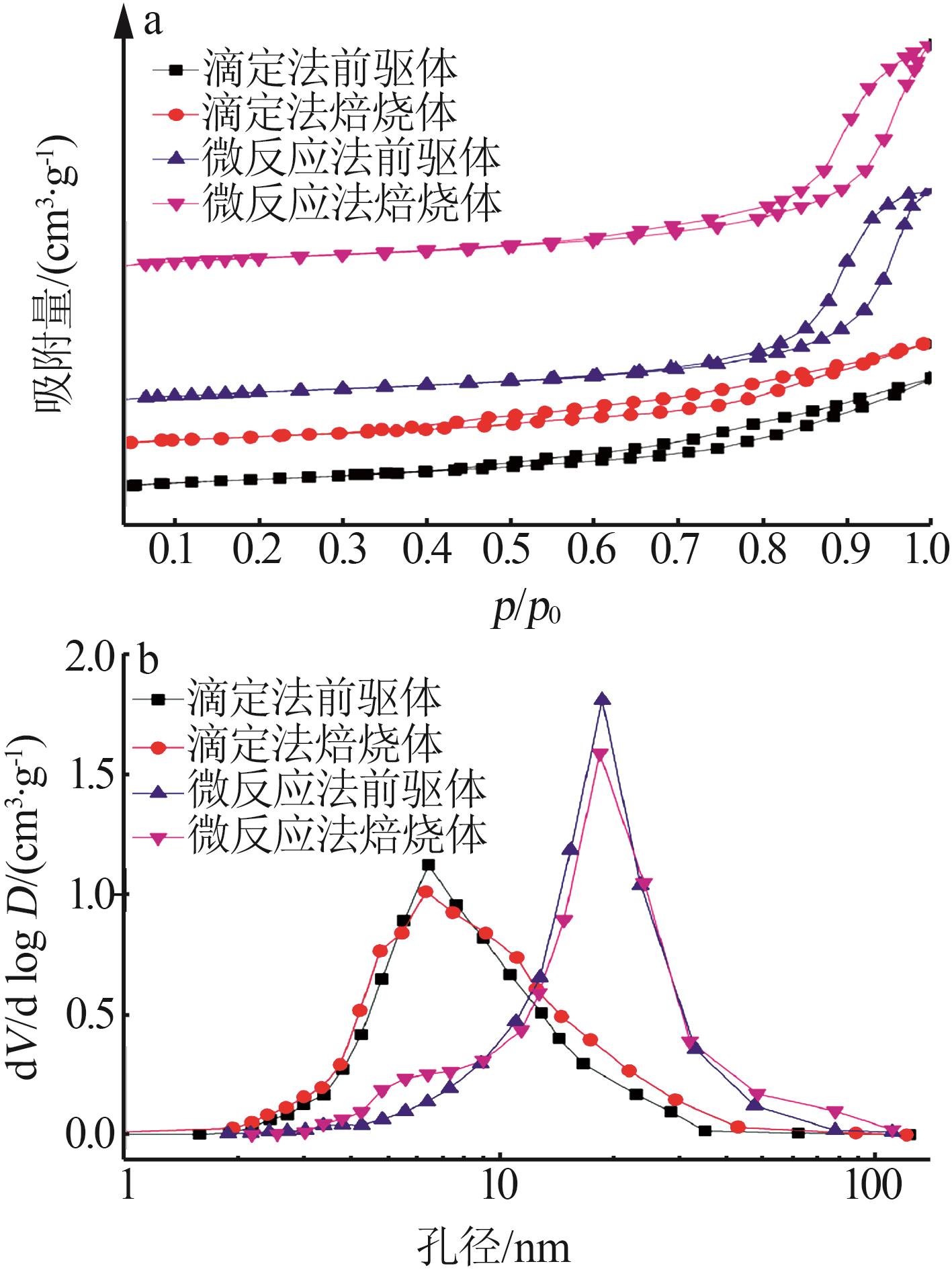
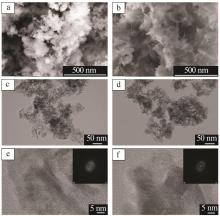
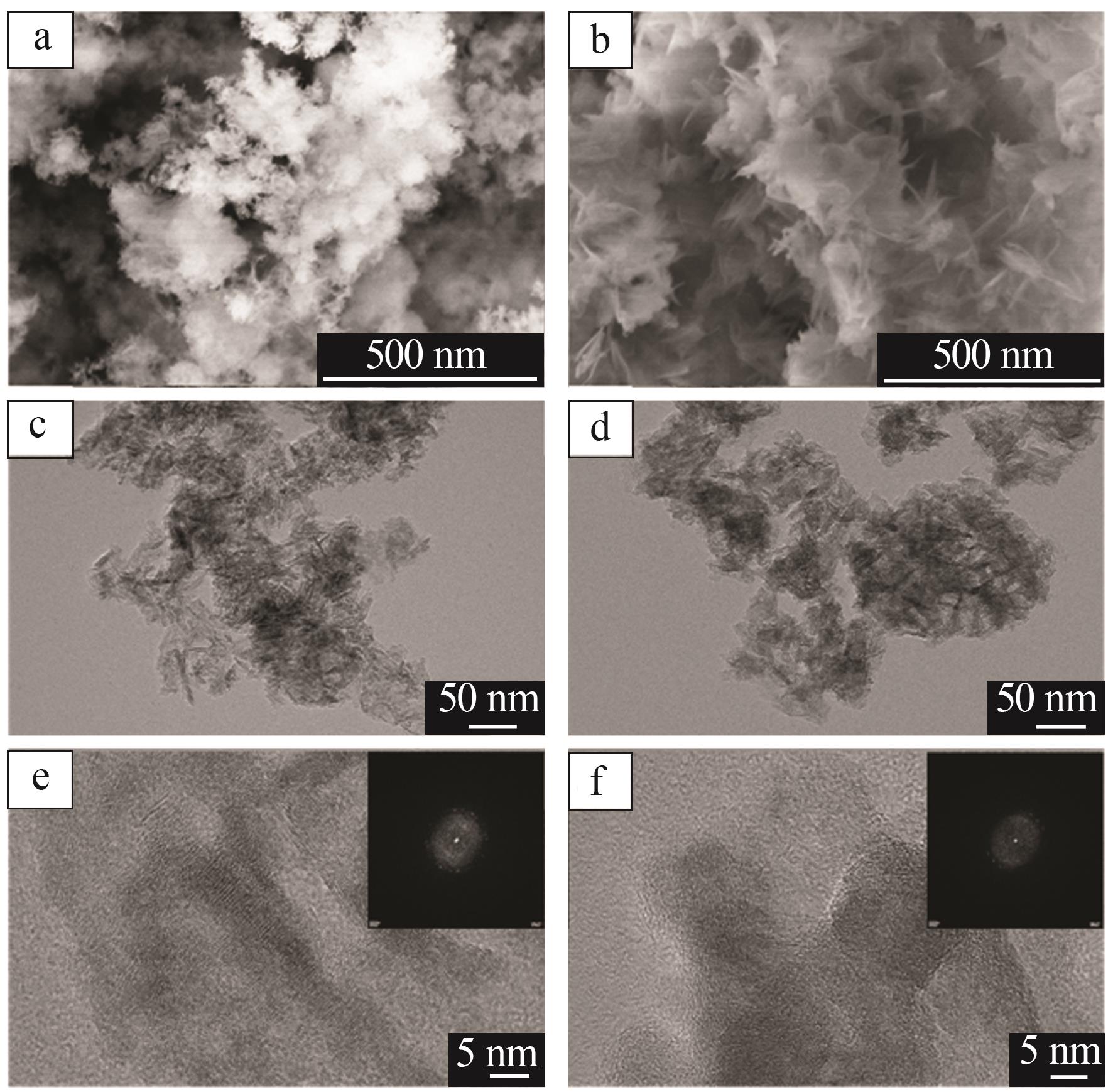
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (9): 66-74.doi: 10.19964/j.issn.1006-4990.2023-0176
• Research & Development • Previous Articles Next Articles
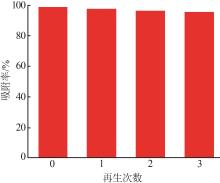
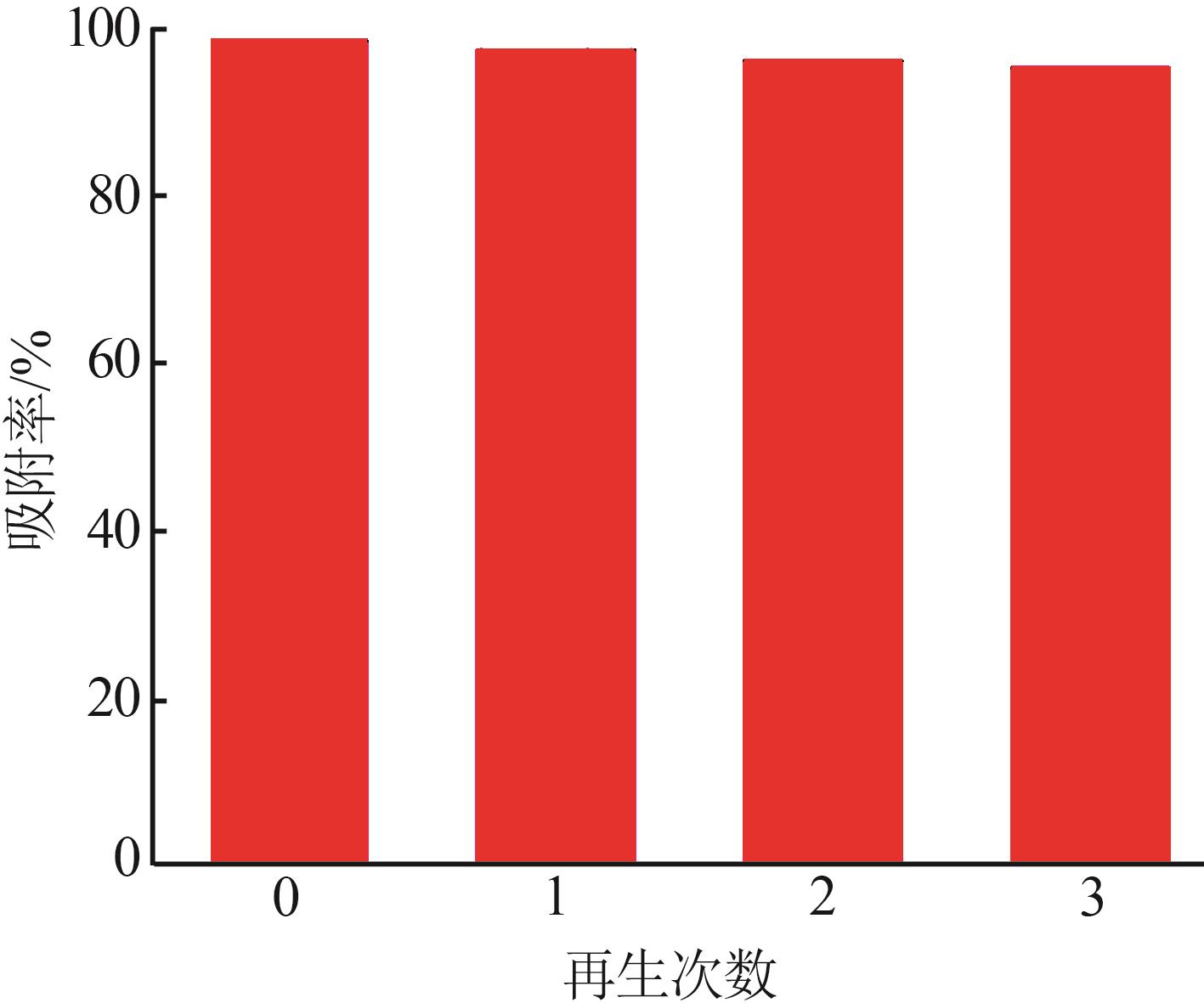
Study on preparation of γ-Al2O3 by microreaction and its properties for methyl orange adsorption
WANG Mengdi( ), LUO Jin, WU Wei, ZHOU Jinghui, WANG Jing, SUN Yanmin, YU Haibin
), LUO Jin, WU Wei, ZHOU Jinghui, WANG Jing, SUN Yanmin, YU Haibin
- CenerTech Tianjin Chemical Research and Design Institute Co. ,Ltd. ,Tianjin 300131,China
-
Received:2023-03-29Online:2023-09-10Published:2023-09-19
CLC Number:
Cite this article
WANG Mengdi, LUO Jin, WU Wei, ZHOU Jinghui, WANG Jing, SUN Yanmin, YU Haibin. Study on preparation of γ-Al2O3 by microreaction and its properties for methyl orange adsorption[J]. Inorganic Chemicals Industry, 2023, 55(9): 66-74.
share this article
| 1 | MA Huanhuan, KONG Aiqun, JI Yanhong,et al.Ultrahigh adsorption capacities for anionic and cationic dyes from wastewater using only chitosan[J].Journal of Cleaner Production,2019,214:89-94. |
| 2 | AHMAD M, YOUSAF M, NASIR A,et al.Porous Eleocharis@MnPE layered hybrid for synergistic adsorption and catalytic biodegradation of toxic azo dyes from industrial wastewater[J].Environmental Science & Technology,2019,53(4):2161-2170. |
| 3 | BEDEKAR P A, BHALKAR B N, PATIL S M,et al.Moringa oleifera-mediated coagulation of textile wastewater and its biodegradation using novel consortium-BBA grown on agricultural waste substratum[J].Environmental Science and Pollution Research International,2016,23(20):20963-20976. |
| 4 | NIDHEESH P V, ZHOU Minghua, OTURAN M A.An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes[J].Chemosphere,2018,197:210-227. |
| 5 | PURNOMO A S, RAHMADINI F D, NAWFA R,et al.The effect of addition of bacterium pseudomonas aeruginosa on biodegradation of methyl orange dye by brown-rot fungus Gloeophyllum trabeum[J].IOP Conference Series:Materials Science and Engineering,2020,980(1):012074. |
| 6 | FAGHIHINEZHAD M, BAGHDADI M, SHAHIN M S,et al.Catalytic ozonation of real textile wastewater by magnetic oxidized g-C3N4 modified with Al2O3 nanoparticles as a novel catalyst[J].Separation and Purification Technology,2022,283:120208. |
| 7 | ZHENG Junfeng, ZHAO Rui, ULIANA A A,et al.Separation of textile wastewater using a highly permeable resveratrol-based loose nanofiltration membrane with excellent anti-fouling performance[J].Chemical Engineering Journal,2022,434:134705. |
| 8 | KARUPPASAMY P, RAMZAN NILOFAR NISHA N, PUGAZHENDHI A,et al.An investigation of transition metal doped TiO2 photocatalysts for the enhanced photocatalytic decoloration of methylene blue dye under visible light irradiation[J].Journal of Environmental Chemical Engineering,2021,9(4):105254. |
| 9 | GUILLOSSOU R, LE ROUX J, MAILLER R,et al.Influence of dissolved organic matter on the removal of 12 organic micropollutants from wastewater effluent by powdered activated carbon adsorption[J].Water Research,2020,172:115487. |
| 10 | VEGA-NEGRON A L, ALAMO-NOLE L, PERALES-PEREZ O,et al.Simultaneous adsorption of cationic and anionic dyes by chitosan/cellulose beads for wastewaters treatment[J].International Journal of Environmental Research,2018,12(1):59-65. |
| 11 | 缪新宇,陆萍,刘双宇,等.光固化氧化铝陶瓷浆料流变性能研究进展[J].硅酸盐通报,2023,42(2):708-718,727. |
| MIAO Xinyu, LU Ping, LIU Shuangyu,et al.Research progress on rheological properties of light-cured alumina ceramic slurry[J].Bulletin of the Chinese Ceramic Society,2023,42(2):708-718,727. | |
| 12 | BARIK M, MISHRA J, DABAS S,et al.Modified boehmite:A choice of catalyst for the selective conversion of glycerol to five-membered dioxolane[J].New Journal of Chemistry,2022,46(2):695-703. |
| 13 | 徐敬尧,周小丽,孙敬会,等.基于复合熔盐低温制备片状α-氧化铝的研究[J].无机盐工业,2023,55(2):73-78. |
| XU Jingyao, ZHOU Xiaoli, SUN Jinghui,et al.Study on preparation of flake α-alumina based on mixed molten salt at low temperature[J].Inorganic Chemicals Industry,2023,55(2):73-78. | |
| 14 | AZARFAR S, NOORBAKHSH F, SALMANI M,et al.Experimental study and characterization of activated alumina adsor-bent[C]//roceedings of Iran International Aluminum Conference(ⅡAC2016).Tehran:Iran Aluminium Research Center Iran University of Science&Technology,2016. |
| 15 | LI Fei, WAN Lisha, WANG Yuqi,et al.Template-free method for the synthesis of high-pore-volume γ-Al2O3 nanofibers in a membrane dispersion microreactor[J].Nanotechnology,2021,32(18):185601. |
| 16 | 杨永钰,田朋,周若辉,等.氢氧化铝活化对水热法制备勃姆石的影响[J].无机盐工业,2022,54(9):55-62 |
| YANG Yongyu, TIAN Peng, ZHOU Ruohui,et al.Effect of gibbsite activation on preparation of boehmite by hydrothermal method[J].Inorganic Chemicals Industry,2022,54(9):55-62 | |
| 17 | 杨文建,孟广莹,李晓云,等.球形氧化铝的制备及其在丙烷脱氢催化剂中的应用[J].无机盐工业,2020,52(6):87-91. |
| YANG Wenjian, MENG Guangying, LI Xiaoyun,et al.Preparation of spherical alumina and its application in PDH[J].Inorganic Chemicals Industry,2020,52(6):87-91. | |
| 18 | GAUTHAM M G, RAO B C, RAMAKRISHNA P A.Combustion synthesis of alumina with possible CO-GENERATION of po-wer[J].International Journal of Hydrogen Energy,2021,46(24):12682-12692. |
| 19 | 于海斌,王梦迪,吴巍,等.微反应器制备催化材料的研究进展及展望[J].无机盐工业,2019,51(9):1-6. |
| YU Haibin, WANG Mengdi, WU Wei,et al.Research progress and prospect of preparation of catalytic materials by microreact-or[J].Inorganic Chemicals Industry,2019,51(9):1-6. | |
| 20 | WAN Yanchun, LIU Yubai, WANG Yujun,et al.Preparation of large-pore-volume γ-alumina nanofibers with a narrow pore size distribution in a membrane dispersion microreactor[J].Industrial & Engineering Chemistry Research,2017,56(31):8888-8894. |
| 21 | YADAV A K, BARANDIARAN M J, DE LA CAL J C.Synthesis of water-borne polymer nanoparticles in a continuous microreactor[J].Chemical Engineering Journal,2012,198/199:191-200. |
| 22 | ALA N, EBRAHIMI M, AFSHAR TAROMI F.A comprehensive study on the synthesis of highly pure dicetyl peroxydicarbonate in a microreactor[J].Chemical Engineering and Processing-Process Intensification,2020,147:107741. |
| 23 | MA Haiyun, JIN Nan, ZHANG Peng,et al.Dynamic characterization of nanoparticles production in a droplet-based continuous flow microreactor[J].Chemical Engineering Research and Design,2019,144:247-257. |
| 24 | HAJJAMI M, GHORBANI-CHOGHAMARANI A, GHAFOURI-NEJAD R,et al.Efficient preparation of boehmite silica dopamine sulfamic acid as a novel nanostructured compound and its application as a catalyst in some organic reactions[J].New Journal of Chemistry,2016,40(4):3066-3074. |
| 25 | ZHANG Huimin, RUAN Yang, FENG Yong,et al.Solvent-free hydrothermal synthesis of gamma-aluminum oxide nanoparticles with selective adsorption of Congo red[J].Journal of Colloid and Interface Science,2019,536:180-188. |
| 26 | SHEK C H, LAI J K L, GU T S,et al.Transformation evolution and infrared absorption spectra of amorphous and crystalline nano-Al2O3 powders[J].Nanostructured Materials,1997,8(5):605-610. |
| 27 | BANERJEE S, DUBEY S, GAUTAM R K,et al.Adsorption characteristics of alumina nanoparticles for the removal of hazardous dye,orange G from aqueous solutions[J].Arabian Journal of Che-mistry,2019,12(8):5339-5354. |
| 28 | FAN Chunyan, ZENG Yonghong, DO D D,et al.A molecular simulation study of adsorption and desorption in closed end slit pores:Is there a hysteresis loop?[J].Chemical Engineering Science,2015,121:313-321. |
| 29 | WU Wei, WAN Zhijian, CHEN Wan,et al.Synthesis of mesoporous alumina with tunable structural properties[J].Microporous and Mesoporous Materials,2015,217:12-20. |
| 30 | HAO Baohong, FANG Keming, XIANG Lan,et al.Synthesization and crystallization mechanism of nano-scale γ-AlOOH with various morphologies[J].International Journal of Minerals,Metallurgy,and Materials,2010,17(3):376-379. |
| 31 | KROKIDIS X, RAYBAUD P, GOBICHON A E,et al.Theoretical study of the dehydration process of boehmite to γ-alumina[J].The Journal of Physical Chemistry B,2001,105(22):5121-5130. |
| 32 | YU Zhiyuan, GUO Chengyu, PANG Xinmei,et al.Coprecipitation synthesis of large-pore-volume γ-alumina nanofibers by two serial membrane dispersion microreactors with a circulating continuous phase[J].Industrial & Engineering Chemistry Research,2023,62(3):1415-1424. |
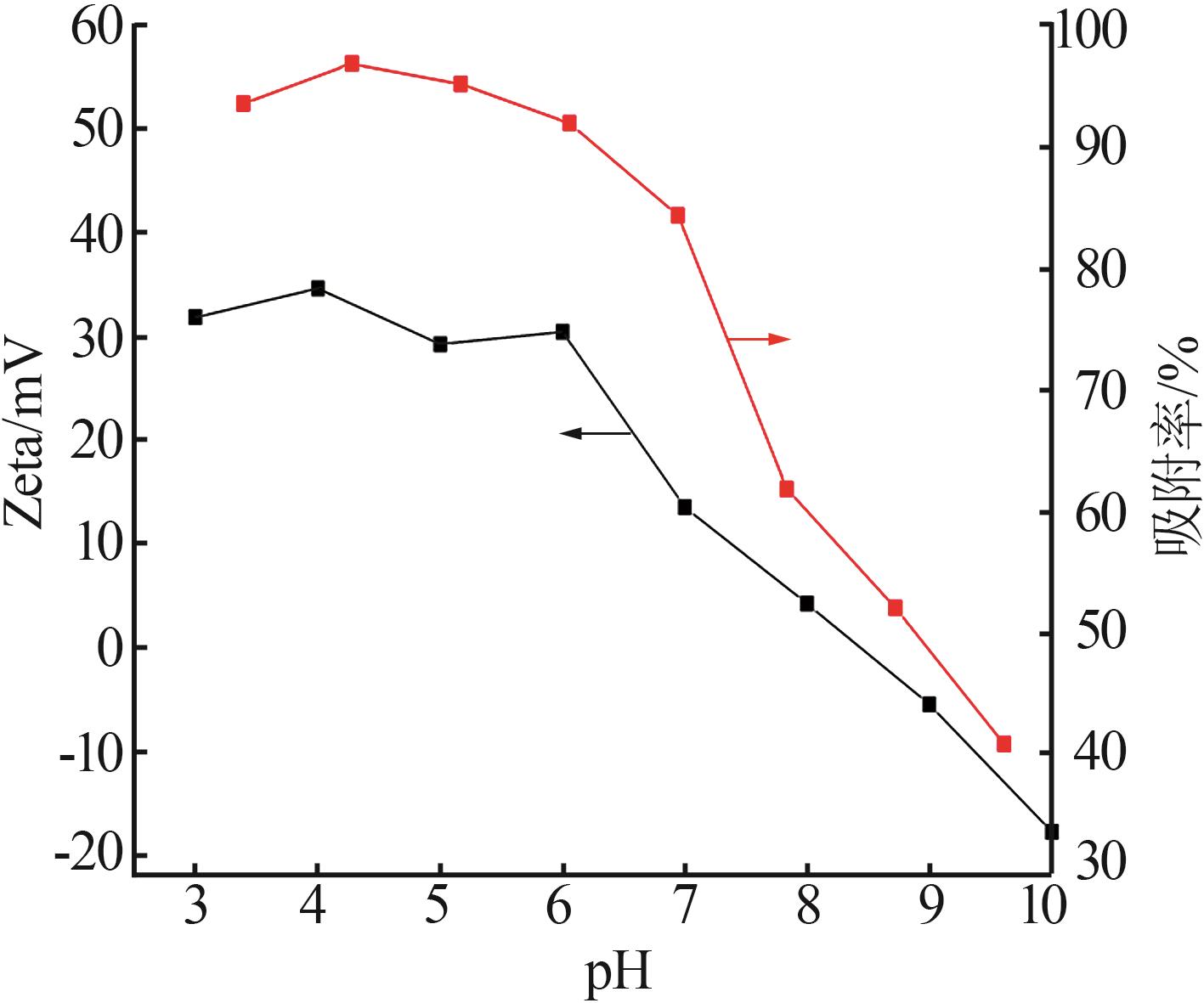
| 33 | KOSMULSKI M.The pH-dependent surface charging and points of zero charge[J].Journal of Colloid and Interface Science,2011,353(1):1-15. |
| 34 | WU Yonghong, YU Qi, XU Hongda,et al.Adsorption behavior and kinetics of methyl orange in water on activated carbon[J].Advanced Materials Research,2012,549:318-321. |
| 35 | PARIDA K M, PRADHAN A C,DAS J,et al.Synthesis and characterization of nano-sized porous gamma-alumina by control precipitation method[J].Materials Chemistry and Physics,2009,113(1):244-248. |
| 36 | WANG Xuhui, CHEN Shuaiqi, SUN Jianchuan,et al.Synthesis of large pore sized mesoporous carbon using alumina-templated strategy for high-performance RhB removal[J].Microporous and Mesoporous Materials,2021,318:110993. |
| 37 | TU Hu, YU Yi, CHEN Jiajia,et al.Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers:Chitosan and cellulose[J].Polymer Chemistry,2017,8(19):2913-2921. |
| 38 | ZHAO Shunzheng, WEN Yanfeng, DU Chengcheng,et al.Introduction of vacancy capture mechanism into defective alumina microspheres for enhanced adsorption of organic dyes[J].Chemical Engineering Journal,2020,402:126180. |
| [1] | ZHAO Chuang, CHEN Zihao, ZHANG Boyu, LI Ben, JIN Fengying, LI Bin, SUN Zhenhai, GUO Chunlei. Study on adsorption and separation performance of molecular sieve adsorbents for different types of diesel [J]. Inorganic Chemicals Industry, 2024, 56(3): 80-85. |
| [2] | LI Yang, LOU Feijian, SUI Xin, LI Keyan, LIU Fei, GUO Xinwen. Preparation of amine-functionalized fumed SiO2 materials and their performance for CO2 adsorption [J]. Inorganic Chemicals Industry, 2024, 56(2): 38-43. |
| [3] | LIU Fujie, HE Qian, SU Long, JIANG Caiyun. Adsorption properties of methylene blue by surface functionalized magnetic biochar with sodium alginate [J]. Inorganic Chemicals Industry, 2024, 56(2): 65-73. |
| [4] | LUO Wenbo, LI Heng, LÜ Jun, YANG Linguang, ZHAO Xingfan, LONG Xiao. Study on recovery of silicon and aluminum from industrial silicon slag [J]. Inorganic Chemicals Industry, 2023, 55(9): 94-99. |
| [5] | KANG Le, JING Maoxiang, LI Donghong, HU Xinyu, JIA Chunyan. Study on preparation and electrochemical performance of lithium aluminate nanorods modified solid electrolyte [J]. Inorganic Chemicals Industry, 2023, 55(8): 65-70. |
| [6] | GUI Changqing, WANG Yajing, LING Changjian, WANG Huaiyou, TANG Zhongfeng. Research progress of preparation and modification of MgO-based CO2 adsorbents [J]. Inorganic Chemicals Industry, 2023, 55(8): 77-83. |
| [7] | PANG Fei, XU Yingrui, CHAI Chunling, SHEN Jingjing, BAI Liguang, ZHAO Xiaodong. Overview on recycling of waste activated alumina in production of hydrogen peroxide by anthraquinone process [J]. Inorganic Chemicals Industry, 2023, 55(6): 1-7. |
| [8] | GAO Fei, DONG Liangfei, GE Yulong, TANG Yuanyuan, LI Fen. Study on preparation of sludge biochar/attapulgite and its adsorption properties [J]. Inorganic Chemicals Industry, 2023, 55(5): 91-99. |
| [9] | XU Jingyao,ZHOU Xiaoli,SUN Jinghui,CAO Alin,QING Peilin. Study on preparation of flake α-alumina based on mixed molten salt at low temperature [J]. Inorganic Chemicals Industry, 2023, 55(2): 73-78. |
| [10] | TIAN Peng, XU Jingang, XU Qianjin, LIU Kunji, PANG Hongchang, NING Guiling. Preparation of nano-alumina slurry and its application in modifying lithium-ion battery cathode material [J]. Inorganic Chemicals Industry, 2023, 55(12): 43-49. |
| [11] | CUI Xiangmei, PAN Tongtong, LUO Qinglong, BIAN Fuxuan, YE Xiushen. Preparation of amino alcohol modified GO/CNTs composite aerogel and boron adsorption from salt lake brines [J]. Inorganic Chemicals Industry, 2023, 55(12): 59-65. |
| [12] | GUO Xueqin, DENG Xiaochuan, ZHU Chaoliang, FU Xin, WANG Ruirui, MA Wanxia, FAN Jie, ZUO Fangtao, QING Binju. Study on preparation of modified diatomite loaded ammonium phosphomolybdate composite adsorbent and its adsorption performance of Cs+ [J]. Inorganic Chemicals Industry, 2023, 55(11): 19-26. |
| [13] | TIAN Peng, ZHOU Ruohui, XU Qianjin, LIU Kunji, PANG Hongchang, NING Guiling. Synthesis and dehydration dynamics of boehmite microcrystalline with different particle sizes [J]. Inorganic Chemicals Industry, 2023, 55(11): 27-36. |
| [14] | JI Ying, ZHANG Ying, HOU Xuechao, ZHU Xiaofeng, JIANG Run, PENG Wenjuan, SUN Guangdong, LÜ Long. Application research of titanium adsorbent of carbonate-type salt lake in Tibet [J]. Inorganic Chemicals Industry, 2023, 55(11): 70-77. |
| [15] | LAI Xianrong, CHEN Zhouqin, SUN Hao, YANG Chao. Pilot study on lithium extraction by adsorption from raw brine of magnesium sulfate subtype salt lakes in Tibet [J]. Inorganic Chemicals Industry, 2023, 55(11): 86-92. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||