Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (10): 136-144.doi: 10.19964/j.issn.1006-4990.2022-0752
• Environment·Health·Safety • Previous Articles Next Articles
Optimization of preparation of ultrafine chromium oxide by carbothermal reduction of sodium dichromate by response surface methodology
ZHANG Zhiqiang1( ), CUI Kangping1(
), CUI Kangping1( ), CHEN Xing1, LI Haiyang1,2
), CHEN Xing1, LI Haiyang1,2
- 1.School of Resources and Environmental Engineering, Hefei University of Technology, Hefei 230009, China
2.Zhongxin Lianke Environmental Technology(Anhui) Co. , Ltd. , Hefei 230009, China
-
Received:2022-12-22Online:2023-10-10Published:2023-10-16 -
Contact:CUI Kangping E-mail:zhangzhiqiang500@163.com;cuikangping@hfut.edu.cn
CLC Number:
Cite this article
ZHANG Zhiqiang, CUI Kangping, CHEN Xing, LI Haiyang. Optimization of preparation of ultrafine chromium oxide by carbothermal reduction of sodium dichromate by response surface methodology[J]. Inorganic Chemicals Industry, 2023, 55(10): 136-144.
share this article
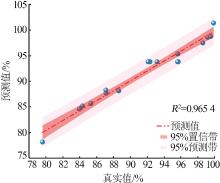
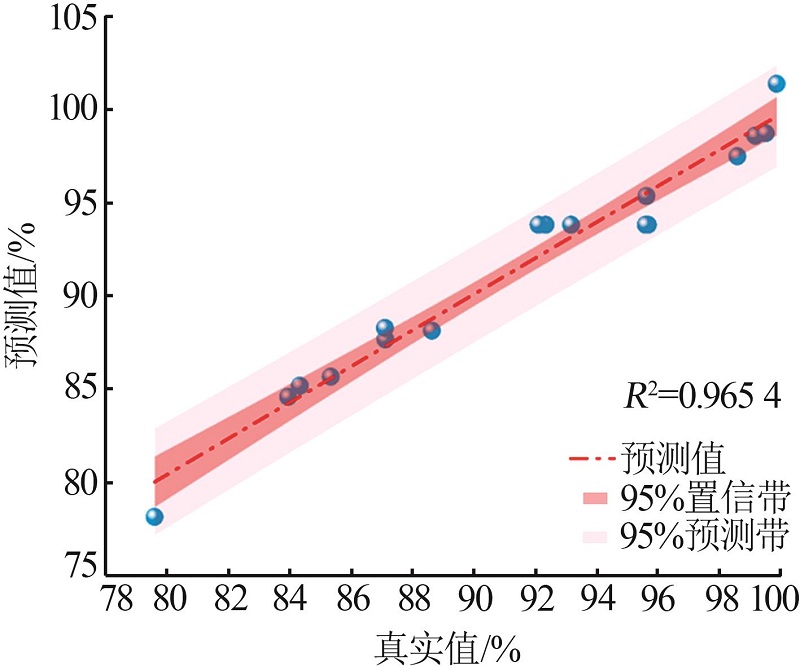
Table 3
Box-Behnken experimental design and results"
| 序号 | 影响因子 | 实验值/ % | 预测值/ % | ||
|---|---|---|---|---|---|
| A | B/h | C/℃ | |||
| 1 | 3 | 2 | 500 | 93.19 | 93.80 |
| 2 | 5 | 2 | 600 | 99.90 | 101.36 |
| 3 | 1 | 3 | 500 | 85.35 | 85.66 |
| 4 | 5 | 3 | 500 | 95.65 | 98.58 |
| 5 | 5 | 1 | 500 | 95.65 | 95.34 |
| 6 | 3 | 2 | 500 | 92.13 | 93.80 |
| 7 | 1 | 1 | 500 | 83.96 | 84.58 |
| 8 | 3 | 3 | 600 | 99.54 | 98.71 |
| 9 | 1 | 2 | 600 | 87.13 | 87.65 |
| 10 | 5 | 2 | 400 | 88.65 | 88.13 |
| 11 | 3 | 2 | 500 | 95.63 | 93.80 |
| 12 | 3 | 2 | 500 | 92.36 | 93.80 |
| 13 | 1 | 2 | 400 | 79.60 | 78.14 |
| 14 | 3 | 1 | 400 | 84.34 | 85.17 |
| 15 | 3 | 1 | 600 | 98.62 | 97.47 |
| 16 | 3 | 3 | 400 | 87.12 | 88.27 |
| 17 | 3 | 2 | 500 | 95.70 | 93.80 |
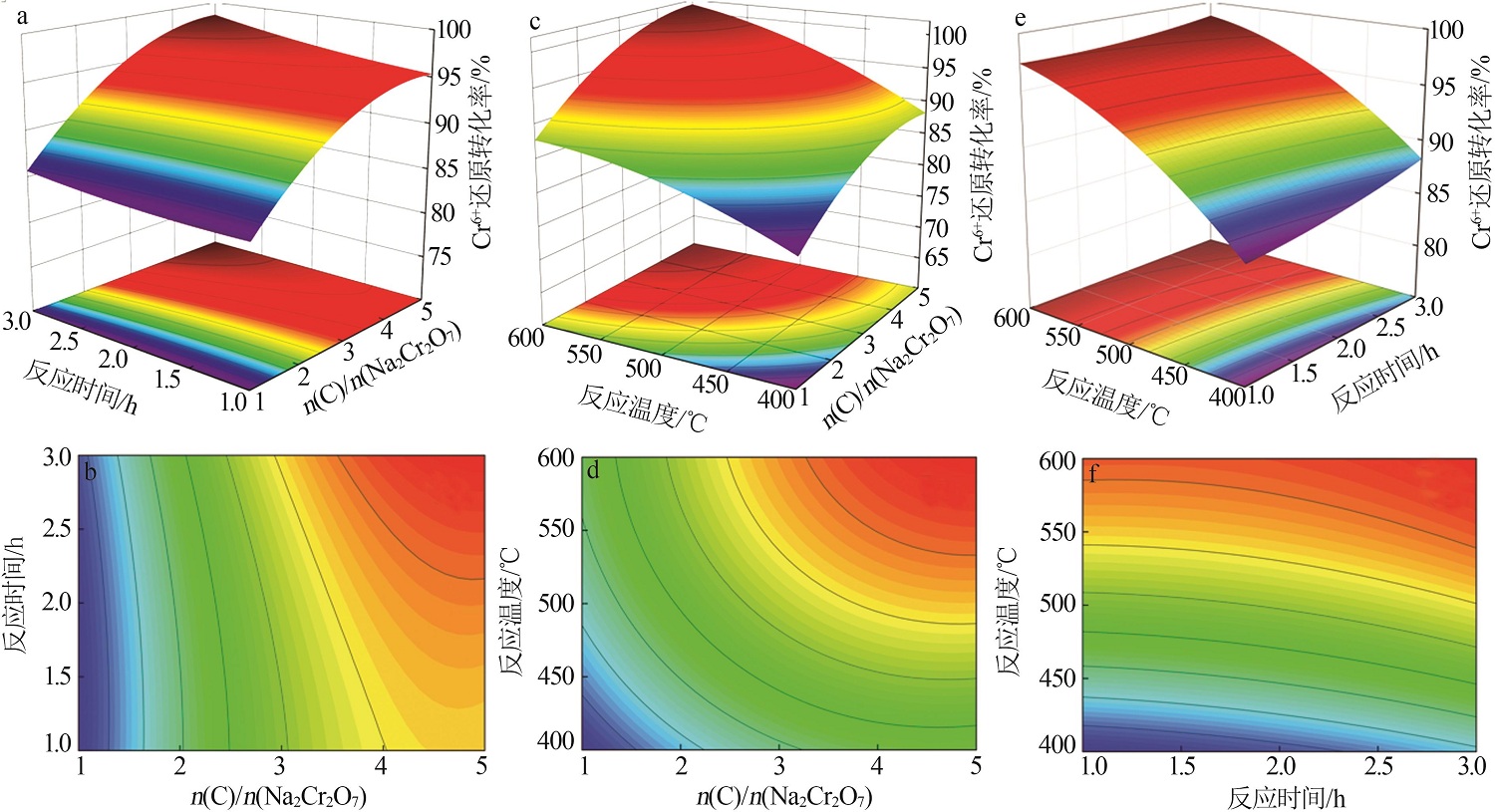
Table 4
Variance analysis of quadratic response surface regression model"
方差 来源 | 平方和 | 自由 度 | 均方差 | F值 | P值 | 显著性 |
|---|---|---|---|---|---|---|
| 模型 | 612.71 | 9 | 68.08 | 21.70 | 0.000 3 | 极显著 |
| A | 280.37 | 1 | 280.37 | 89.39 | <0.000 1 | 极显著 |
| B | 9.33 | 1 | 9.33 | 2.97 | 0.128 2 | 不显著 |
| C | 258.55 | 1 | 258.55 | 82.43 | <0.000 1 | 极显著 |
| AB | 1.17 | 1 | 1.17 | 0.371 9 | 0.561 3 | 不显著 |
| AC | 3.46 | 1 | 3.46 | 1.10 | 0.328 5 | 不显著 |
| BC | 0.864 9 | 1 | 0.864 9 | 0.275 7 | 0.615 7 | 不显著 |
| A2 | 42.40 | 1 | 42.40 | 13.52 | 0.007 9 | 极显著 |
| B2 | 0.713 0 | 1 | 0.713 0 | 0.227 3 | 0.648 1 | 不显著 |
| C2 | 13.77 | 1 | 13.77 | 4.39 | 0.047 4 | 显著 |
| 残差 | 21.96 | 7 | 3.14 | — | — | |
| 失拟项 | 9.76 | 3 | 3.25 | 1.07 | 0.456 5 | 不显著 |
| 纯误差 | 12.19 | 4 | 3.05 | — | — | |
| 总离差 | 634.66 | 16 | — | — | — |
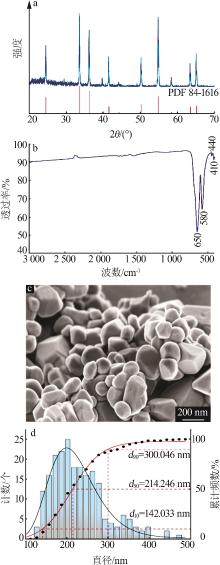
Table 7
Composition of Cr2O3 product and industrial grade Cr2O3 index requirements"
| 指标 | w(Cr2O3)/ % | w(水溶性铬)/% | w(水分)/ % | w(水溶物)/ % | pH(100 g/L 悬浮液) | 吸油量/ g | w(粒径>0.045 mm 粒子)/% | |
|---|---|---|---|---|---|---|---|---|
| Ⅰ类 | 优等品 | ≥99.0 | ≤0.005 | ≤0.15 | ≤0.1 | 6~8 | 15~25 | ≤0.1 |
| 一等品 | ≥99.0 | ≤0.03 | ≤0.15 | ≤0.3 | 5~8 | 15~25 | ≤0.2 | |
| 合格品 | ≥98.0 | ≤0.03 | ≤0.3 | ≤0.4 | 5~8 | 15~25 | ≤0.3 | |
| Ⅱ类 | 优等品 | ≥99.0 | ≤0.005 | ≤0.15 | ≤0.2 | — | ≤20 | ≤0.2 |
| 一等品 | ≥99.0 | ≤0.03 | ≤0.15 | ≤0.3 | — | ≤25 | ≤0.2 | |
| 合格品 | ≥98.0 | ≤0.03 | ≤0.3 | ≤0.5 | — | ≤25 | — | |
| 本文产品 | 99.53 | 0.004 | 0.0 | 0.1 | 7.5 | 23 | 0.1 | |
| 1 | 丁翼.铬化合物生产与应用[M].北京:化学工业出版社,2003. |
| 2 | 徐志恒.等离子溅射沉积Cr2O3的冲刷腐蚀性能的研究[D].武汉:武汉工程大学,2017. |
| XU Zhiheng.Investigation on erosion-corrosion behaviours of sputter-deposited Cr2O3 coating prepared by double cathode plasma technique[D].Wuhan:Wuhan Institute of Technology,2017. | |
| 3 | 佟小宇.论氧化铬绿在涂料生产中的应用[J].化学工程与装备,2010(2):51-52. |
| 4 | WANG Yang, GAO Weizhe, WANG Kangzhou,et al.Boosting the synthesis of value-added aromatics directly from syngas via a Cr2O3 and Ga doped zeolite capsule catalyst[J].Chemical Science,2021,12(22):7786-7792. |
| 5 | GUAN Yuepeng, LIU Xiaojun, AKHTAR N,et al.Cr2O3 nanoparticle decorated carbon nanofibers derived from solid leather wastes for high performance lithium-sulfur battery separator coating[J].Journal of the Electrochemical Society,2019,166(8):A1671-A1676. |
| 6 | JANTZEN C M, IMRICH K J, BROWN K G,et al.High chrome refractory characterization:Part I.Impact of melt reduction/oxidation on the corrosion mechanism[J].International Journal of Applied Glass Science,2015,6(2):137-157. |
| 7 | SINGLA M K, NIJHAWAN P, OBEROI A S.Hydrogen fuel and fuel cell technology for cleaner future:A review[J].Environmental Science and Pollution Research,2021,28(13):15607-15626. |
| 8 | ZHANG Meng, XIONG Zhengwei, JIA Jinzhi,et al.Improving electrochemical performance of hollow Cr2O3/CrN nanoshells as electrode materials for supercapacitors[J].Journal of Electroanalytical Chemistry,2020,856:113696. |
| 9 | HARYŃSKI Ł, OLEJNIK A, KARCZEWSKI J,et al.Linking optical and electronic properties to photoresponse of heterojunctions based on titania nanotubes and chromium,molybdenum,and tungsten oxides[J].Optical Materials,2022,134:113183. |
| 10 | MANSMANN M, RAMBOLD W.Production of improved chromium oxide green pigment:US,4067747[P].1978-01-10. |
| 11 | MANSMANN M, RAMBOLD W.Chromium oxide pigment from sodium chromate dihydrate plus ammonium salt:US,4040860[P]. 1977-08-09. |
| 12 | FOUAD N E.Non-isothermal kinetics of CrO3 decomposition pathways in air[J].Journal of Thermal Analysis,1996,46(5):1271-1282. |
| 13 | AVENA M J, GIACOMELLI C E, DE PAULI C P.Formation of Cr(Ⅲ) hydroxides from chrome alum solutions:1.Precipitation of active chromium hydroxide [J].Journal of Colloid and Interface Science,1996,180(2):428-435. |
| 14 | 陈霖,何莉萍,赖琼林,等.纳米氧化铬制备方法及其进展[J].材料导报,2005,19(z2):144-146. |
| CHEN Lin, HE Liping, LAI Qionglin,et al.Preparation methods and their progress of nanometer-sized Cr2O3 [J].Materials Reports,2005,19(z2):144-146. | |
| 15 | DURANOĞLU D, BUYRUKLARDAN KAYA İ G, BEKER U,et al.Synthesis and adsorption properties of polymeric and polymer-based hybrid adsorbent for hexavalent chromium removal[J].Chemical Engineering Journal,2012,181-182:103-112. |
| 16 | 叶雪梅.铬酸钠电解液制备三氧化二铬的实验研究[D].西宁:中国科学院大学(中国科学院青海盐湖研究所),2019. |
| YE Xuemei.Preparation of chromium oxide from sodium chromate electrolytes[D].Xining:Qinghai Institute of Salt Lakes,Chinese Academy of Sciences,2019. | |
| 17 | PEI Zhenzhao, GAO Xiang, ZHANG Yunxia,et al.Hydrothermal synthesis of large sized sphere:Like polyhedrons of Cr2O3 under the assistance of surfactant cetyltrimethylammonium bromide (CTAB)[J].Materials Letters,2014,116:215-218. |
| 18 | LI Ping, XU Hongbin, ZHENG Shili,et al.A green process to prepare chromic oxide green pigment[J].Environmental Science & Technology,2008,42(19):7231-7235. |
| 19 | 魏潇,廖辉伟,陈宁,等.铬酸钠碳化母液制备氢氧化铬工艺优化及三氧化二铬粒度分布的影响[J].无机盐工业,2019,51(11):36-41. |
| WEI Xiao, LIAO Huiwei, CHEN Ning,et al.Process optimization of preparation of chromium hydroxide from sodium chromate carbonization mother liquor and effect of particle size distribution of chromium trioxide[J].Inorganic Chemicals Industry,2019,51(11):36-41. | |
| 20 | LIANG Shuting, ZHANG Hongling, LUO Minting,et al.Colour performance investigation of a Cr2O3 green pigment prepared via the thermal decomposition of CrOOH[J].Ceramics International,2014,40(3):4367-4373. |
| 21 | GUAN Su, DENG Feng, HUANG Siqi,et al.Optimization of magnetic field-assisted ultrasonication for the disintegration of waste activated sludge using Box-Behnken design with response surface methodology[J].Ultrasonics Sonochemistry,2017,38:9-18. |
| 22 | 石月,彭湃,刘艳丽,等.UV/Fe2+/过硫酸盐降解噻虫啉的响应曲面法优化研究[J].中国环境科学,2021,41(11):5153-5159. |
| SHI Yue, PENG Pai, LIU Yanli,et al.Degradation of thiacloprid via UV/Fe2+/persulfate system:Optimization using response surface methodology[J].China Environmental Science,2021,41(11):5153-5159. |
| [1] | ZHANG Conghua, YAN Wenbin, XIAO Jiajun, ZHAO Ke, PENG Shangquan, WEI Yuhong. Reductive leaching technology of manganese anode slag using tartaric acid as reducing agent optimized by RSM [J]. Inorganic Chemicals Industry, 2023, 55(9): 106-113. |
| [2] | WANG Yingnan, SHENG Linlin, HUANG Juan, HUANG Zhanbin. Study on adsorption performance of lead from water by coal-fired slag [J]. Inorganic Chemicals Industry, 2023, 55(8): 109-115. |
| [3] | TENG Jiayang, FENG Qingge, ZHANG Xuan, QIN Fanghong, FENG Jinghang, HU Jiawen, CHEN Chaohong. Study on preparation of pseudo-boehmite from aluminum dross resource treatment [J]. Inorganic Chemicals Industry, 2023, 55(11): 130-138. |
| [4] | XU Enhao, WU Kaipeng. Research progress of preparation and application of nano-sized chromium oxide [J]. Inorganic Chemicals Industry, 2023, 55(10): 24-34. |
| [5] | FANG Weicheng,CHENG Xingxing,SUN Changrong. Optimization of preparation of sludge/fly ash composite ceramsite filler materials by response surface methodology [J]. Inorganic Chemicals Industry, 2022, 54(9): 119-125. |
| [6] | FU Ziqi, ZHANG Cheng, SHENG Yong, JI Lijun. Study on preparation of phosphoric acid by leaching fluoride residue from wet-process phosphoric acid with organic solvents [J]. Inorganic Chemicals Industry, 2022, 54(7): 129-134. |
| [7] | LI Yajiao,ZHAO Yiwei,JU Kai,TANG Renlong,LI Longqing,SHAO Xiaoping,ZHANG Gaofeng,REN Wuang. Study on optimization of extraction conditions in process of determination of ammonia content in fly ash based on response surface method [J]. Inorganic Chemicals Industry, 2022, 54(4): 145-151. |
| [8] | Huang Li,Wang Hu,Zong Yuhao,Chang Zhengfeng,Liu Yang,Li Jinke,Han Pei. Study on deactivation effect of chromium on industrial V-Mo/Ti De-NOx catalyst [J]. Inorganic Chemicals Industry, 2021, 53(8): 112-116. |
| [9] | Liu Jijun,Lin Hongmei,Hu Guorong,Peng Zhongdong. Study on new process of producing chromium salt with ferrochrome alloy [J]. Inorganic Chemicals Industry, 2021, 53(6): 156-159. |
| [10] | Huang Jiming,Liu Runqing,Wu Sizhan,Song Juan. Optimization of extraction process of chlorination roasting-water leaching process for low-grade rhodochrosite by response surface methodology [J]. Inorganic Chemicals Industry, 2019, 51(3): 34-37. |
| [11] | Wei Xiao1,Liao Huiwei1,Chen Ning2,Dong Mingfu2,Huang Xiandong2,Huang Yuxi2. Process optimization of preparation of chromium hydroxide from sodium chromate carbonization mother liquor and effect of particle size distribution of chromium trioxide [J]. Inorganic Chemicals Industry, 2019, 51(11): 36-41. |
| [12] | Liu Yuxin1,Shi Fengjuan2,Lü Yaohui1,Wei Shicheng1,Wang Yujiang1,He Dongyu1,Wang Wenyu1. Progress in preparation of nano?鄄sized or porous chromium oxide particles [J]. Inorganic Chemicals Industry, 2019, 51(10): 1-6. |
| [13] | Tian Sen1,2,3,Ye Xuemei1,2,3,Feng Haitao1,2,Dong Yaping1,2,Li Wu1,2,Zhang Bo1,2,Li Bo1,2. Effect of temperature on preparation of chromium oxide by thermal decomposition of ammonium dichromate [J]. Inorganic Chemicals Industry, 2019, 51(10): 60-63. |
| [14] | CHEN Bo, SONG Xing-Fu, XU Yan-Xia, SUN Yu-Zhu, YU Jian-Guo. Optimization of preparation of calcium carbonate from continuous crystallization process of ammonium carbonate and conversion of calcium sulfate by response surface methodology [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 18-. |
| [15] | JIANG Qing-Ping, CHEN Ning, ZHANG Guo-Qing, CHEN Yan, DU Jian-Bo. Preparation of chromium oxide green by reduction of chromium-containing waste liquid with urea hydrothermal method [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(4): 51-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||