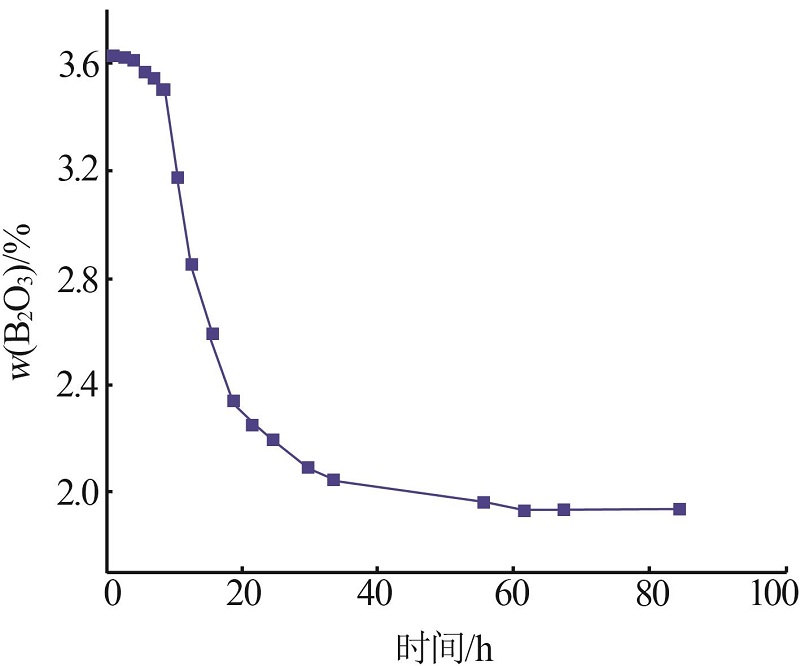
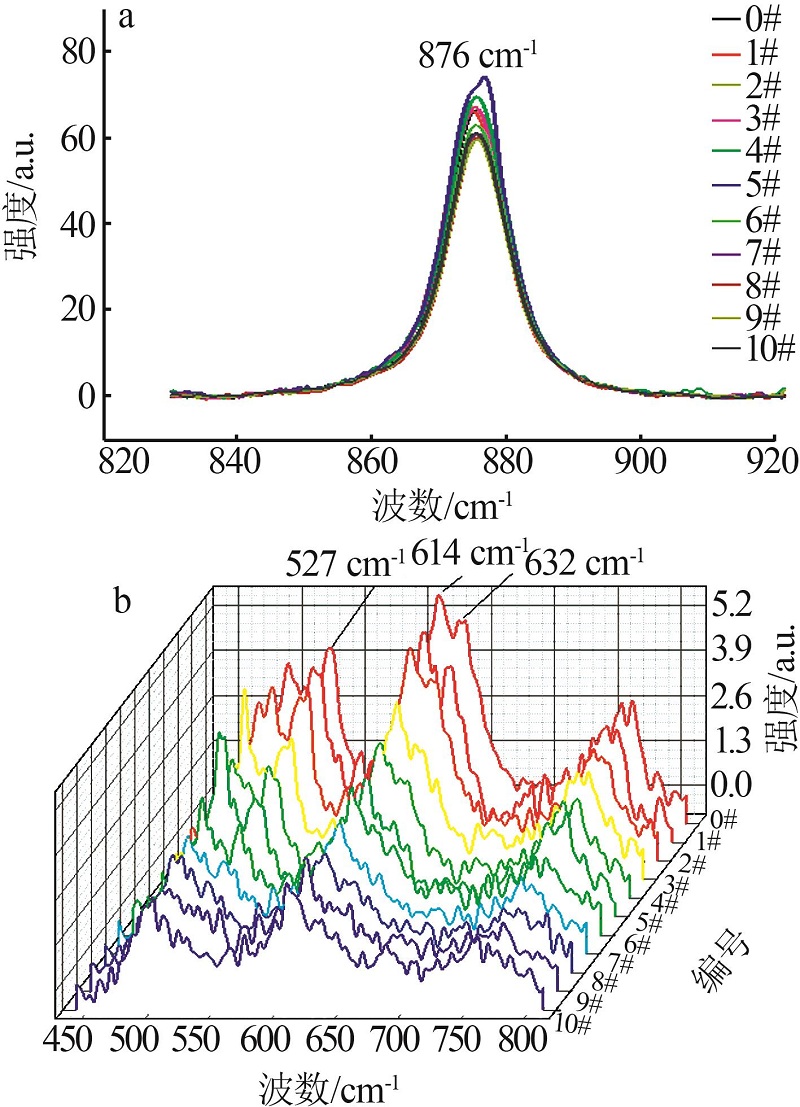
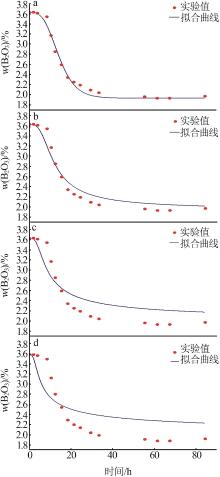
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (10): 56-62.doi: 10.19964/j.issn.1006-4990.2022-0734
• Research & Development • Previous Articles Next Articles
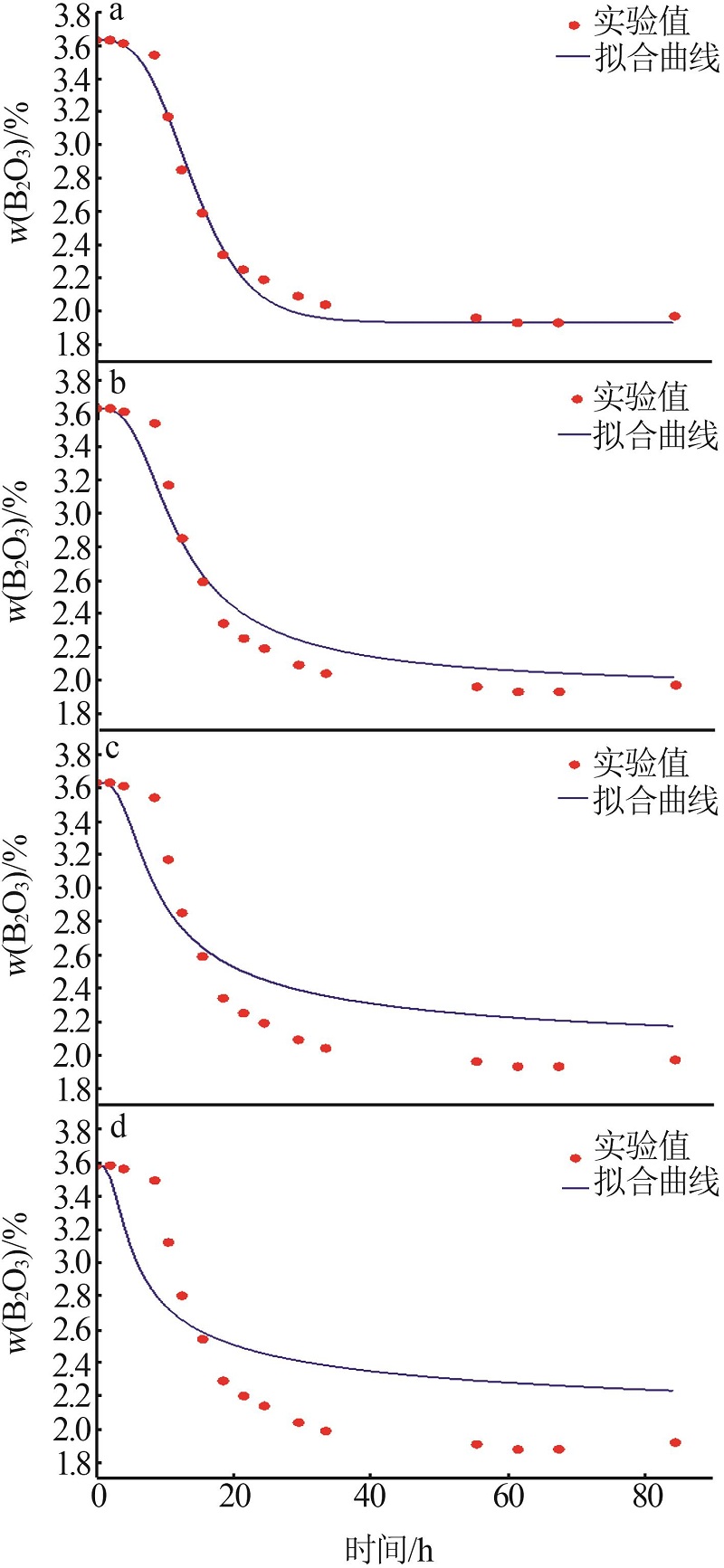
Study on crystallization mechanism and kinetics of macallisterite synthesized with bischofite from salt lake
PENG Jiaoyu1,2( ), TAN Yuqin3, YANG Keli1,2, DONG Yaping1,2, ZHANG Bo1,4, LI Wu1,4
), TAN Yuqin3, YANG Keli1,2, DONG Yaping1,2, ZHANG Bo1,4, LI Wu1,4
- 1.Key Laboratory of Comprehensive and Highly Efficient Utilization of Salt Lake Resources,Qinghai Institute of Salt Lakes,Chinese Academy of Sciences,Xining 810008,China
2.Qinghai Engineering and Technology Research Center of Comprehensive Utilization of Salt Lake Resources,Xining 810008,China
3.Qinghai Normal University,Xining 810016,China
4.Key Laboratory of Salt Lake Resources Chemistry of Qinghai Province,Xining 810008,China
-
Received:2022-12-12Online:2023-10-10Published:2023-10-16
CLC Number:
Cite this article
PENG Jiaoyu, TAN Yuqin, YANG Keli, DONG Yaping, ZHANG Bo, LI Wu. Study on crystallization mechanism and kinetics of macallisterite synthesized with bischofite from salt lake[J]. Inorganic Chemicals Industry, 2023, 55(10): 56-62.
share this article
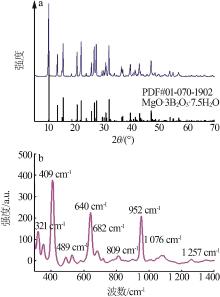
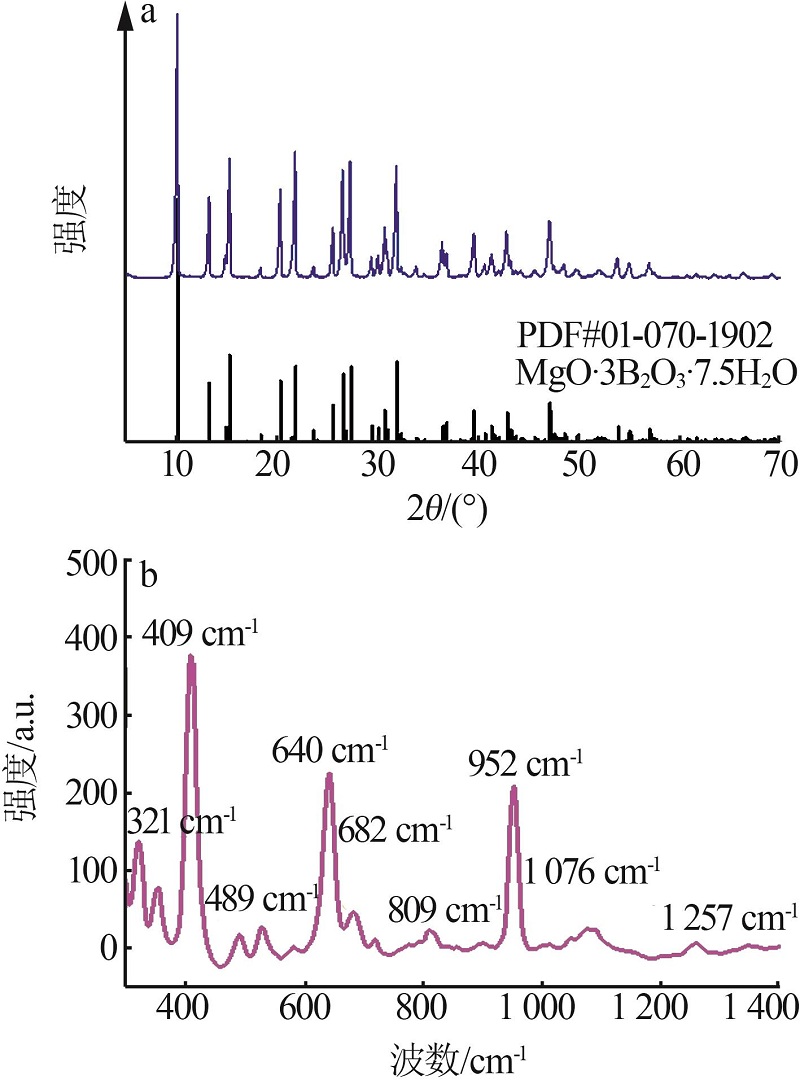
Table 1
Effects of temperature and mole ratio on solid phase"
温度/ ℃ | n(NaB5O8):n(MgCl2) | XRD鉴定 |
|---|---|---|
| 25 | 1:4 | MgO·3B2O3·7.5H2O |
| 1:8 | MgO·3B2O3·7.5H2O | |
| 1:12 | MgO·3B2O3·7.5H2O+MgCl2·6H2O+H3BO3(少量) | |
| 1:16 | MgO·3B2O3·7.5H2O+MgCl2·6H2O+H3BO3(少量) | |
| 40 | 1:4 | MgO·3B2O3·7.5H2O |
| 1:8 | MgO·3B2O3·7.5H2O | |
| 1:12 | MgO·2B2O3·9H2O+MgCl2·6H2O+NaCl(少量) | |
| 1:16 | MgO·3B2O3·7.5H2O+MgO·3B2O3·6H2O+H3BO3 (少量) | |
| 60 | 1:4 | MgO·3B2O3·7.5H2O |
| 1:8 | MgO·3B2O3·7.5H2O |
| 1 | 谢金刚,葛倪林,王中立,等.硼酸盐化合物的研究进展[J].安徽化工,2022,48(4):1-4. |
| XIE Jingang, GE Nilin, WANG Zhongli,et al.Research progress on boron compounds[J].Anhui Chemical Industry,2022,48(4):1-4. | |
| 2 | 刘润清.基于BO3基团稀土硼酸盐非线性光学晶体的合成及性能研究[D].天津:天津理工大学,2022. |
| LIU Runqing.Syntheses and properties of rare earth borate nonlinear optical crystals based on BO3 groups[D].Tianjin:Tianjin University of Technology,2022. | |
| 3 | 杨军,郭俐聪,郑学家.多功能金属硼酸盐合成与应用[M].北京:化学工业出版社,2018:2-13. |
| 4 | GAO Xianshu, ZHOU Ping, QIN Xiaoliang,et al.The effects of magnesium borate whiskers on the mechanical performance of oil well cement[J].Advances in Materials Science and Engineering,2022,2022:8442648. |
| 5 | SAFFARI H R M, SOLTANI R, ALAEI M,et al.Tribological properties of water-based drilling fluids with borate nanoparticles as lubricant additives[J].Journal of Petroleum Science and Engineering,2018,171:253-259. |
| 6 | 李臻,杨丽庭,李彦涛,等.硼酸盐对阻燃PP复合材料性能的影响[J].华南师范大学学报(自然科学版),2021,53(2):35-43. |
| LI Zhen, YANG Liting, LI Yantao,et al.The effect of borate on the properties of flame-retardant PP composites[J].Journal of South China Normal University(Natural Science Edition),2021,53(2):35-43. | |
| 7 | 刘婴.新型金属硼酸盐的设计合成、表征及性质研究[D].广州:广东工业大学,2021. |
| LIU Ying.Design synthesis,characterization and property research of novel metal borates[D].Guangzhou:Guangdong University of Technology,2021. | |
| 8 | 李娜,刘志启,郭凡,等.镁源种类对制备碱式硼酸镁的影响[J].盐湖研究,2020,28(3):66-70. |
| LI Na, LIU Zhiqi, GUO Fan,et al.Effect of magnesium sources on preparation of magnesium borate hydroxide[J].Journal of Salt Lake Research,2020,28(3):66-70. | |
| 9 | 胡应喜,文帅,郭瑞,等.硼酸镁微波水热合成、表征及发光性能[J].无机盐工业,2017,49(12):16-19. |
| HU Yingxi, WEN Shuai, GUO Rui,et al.Microwave hydrothermal synthesis,characterization and photoluminescence properties of magnesium borate[J].Inorganic Chemicals Industry,2017,49(12):16-19. | |
| 10 | KADARI A, CHIKHAOUI R, EL-MESADY I A.Enhancement of structural and optical properties of synthesized Dy3+-Mn2+ doped and co-doped magnesium borate MgB4O7 [J].Optical Materials,2022,133:112929. |
| 11 | BAKHSH M, YASUDA H, AHMAD N,et al.Zinc-doped magnesium borate glass:A potential thermoluminescence dosimeter for extended range of dosimetric applications[J].Applied Sciences,2022,12(15):7491. |
| 12 | HE Chao, SHUI Anze, MA Juan,et al. In situ growth magnesium borate whiskers and synthesis of porous ceramics for sound-absorbing[J].Ceramics International,2020,46(18):29339-29343. |
| 13 | ZOU Sai, DANG Li, LI Ping,et al.Organic-inorganic modification of magnesium borate rod by layered double hydroxide and 3-aminopropyltriethoxysilane and its effect on the properties of epoxy resin[J].Polymers,2022,14(17):3661. |
| 14 | DEMIREL B, KıLıÇ E, YARAŞ A,et al.Effects of magnesium borate on the mechanical performance,thermal and chemical degradation of polyethylene terephthalate packaging material[J].Journal of Plastic Film & Sheeting,2022,38(4):589-607. |
| 15 | 高世扬,赵金福,薛方山,等.盐卤硼酸盐化学Ⅱ.从含硼浓缩氯化镁卤水中析出的六硼酸镁MgO·3B2O3·7½H2O[J].化学学报,1983,41(3):217-221. |
| GAO Shiyang, ZHAO Jinfu, XUE Fangshan,et al.The chemistry of borate in salt lake brine Ⅱ.Hydrated Mg-borate crystallizing out from concentrated magnesium chloride brine[J].Acta Chimica Sinica,1983,41(3):217-221. | |
| 16 | 曲一華,韓蔚田,錢自強,等.三方硼鎂石:一种新硼酸盐矿物[J].地质学报,1965,39(3):298-305. |
| CHU I-Hwa, HAN Wei-Tien, CHIEN Tse-Chang,et al.Trigonomagneborite:a new borate mineral[J].Acta Geological Sinica,1965,39(3):298-305. | |
| 17 | 克山А .Л.硼酸盐在水溶液中的合成及其研究[M].成思危,译.北京:科学出版社,1962:91-92. |
| 18 | 孟令宗,邓天龙,段超文,等.三方硼镁石合成方法研究[J].世界科技研究与发展,2010,32(6):825-826. |
| MENG Lingzong, DENG Tianlong, DUAN Chaowen,et al.A synthetic method for mcallisterite[J].World Sci-Tech R&D,2010,32(6):825-826. | |
| 19 | MOROYDOR DERUN E, SENBERBER F T.Characterization and thermal dehydration kinetics of highly crystalline mcallisterite,synthesized at low temperatures[J].The Scientific World Journal,2014,2014:985185. |
| 20 | KIPCAK A S, YILDIRIM M, AYDIN YUKSEL S,et al.The synthesis and physical properties of magnesium borate mineral of admontite synthesized from sodium borates[J].Advances in Materials Science and Engineering,2014,2014:1-9. |
| 21 | YILDIRIM M, KIPCAK A S, DERUN E M.Sonochemical-assisted magnesium borate synthesis from different boron sources[J].Polish Journal of Chemical Technology,2017,19(1):81-88. |
| 22 | 段雪,林彦军,项顼,等.青海盐湖镁锂资源综合利用的建议与实践[J].青海科技,2022,29(3):4-10. |
| DUAN Xue, LIN Yanjun, XIANG Xu,et al.Suggestion and practice on comprehensive utilization of magnesium and lithium resources in Qinghai salt lakes[J].Qinghai Science and Technology,2022,29(3):4-10. | |
| 23 | 邓良明,蒋志税,李一凰.盐湖水氯镁石复合精制实验研究[J].无机盐工业,2022,54(10):52-56. |
| DENG Liangming, JIANG Zhishui, LI Yihuang.Experimental study on compound refining of salt lake bischofite[J].Inorganic Chemicals Industry,2022,54(10):52-56. | |
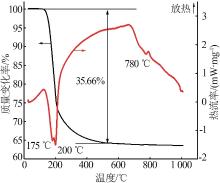
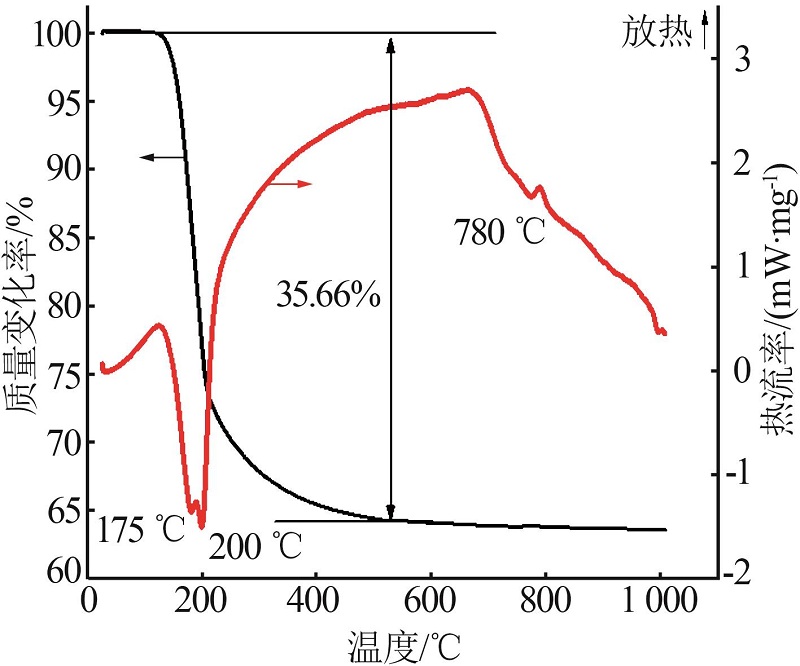
| 24 | PENG Jiaoyu, CHEN Jing, DONG Yaping,et al.Investigations on Mg-borate kinetics and mechanisms during evaporation,dilution and crystallization by Raman spectroscopy[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2018,199:367-375. |
| 25 | PENG Jiaoyu, ZHANG Bo, CHEN Jing,et al.Crystallization kinetics of Mg-borates precipitating from diluted boron-containing brine of Da Qaidam saline lake [J].Chinese Journal of Inorganic Chemistry,2019,35(10):1821-1833. |
| 26 | NIELSEN A E.Kinetics of precipitation[M].New York:Macmil-lan,1964. |
| [1] | FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. |
| [2] | ZHOU Qiang, WU Bin, CHEN Kui, JI Lijun, WU Yanyang. Study on thermal decomposition kinetic mechanism and calcination process of phosphorus tailings [J]. Inorganic Chemicals Industry, 2023, 55(3): 47-54. |
| [3] | TIAN Xiaoli, LI Zhixun, FENG Runtang, ZHANG Jie, ZHENG Quanfu, SHI Xuwu, DU Yongbin. Study on thermal decomposition behavior of Tibetan Kamado microcrystalline magnesite [J]. Inorganic Chemicals Industry, 2023, 55(3): 60-65. |
| [4] | ZHANG Xing,XU Jie,WANG Zibing,HOU Peng,HE Long,LIU Huan. Effect of feedstock particle size on kinetics of limestone thermal decomposition reaction [J]. Inorganic Chemicals Industry, 2023, 55(2): 79-84. |
| [5] | DING Ning, ZHANG Jian, PING Qingwei, SHENG Xueru, LI Na. Study on adsorption and release properties of matrine by magnesium-modified diatomite [J]. Inorganic Chemicals Industry, 2023, 55(11): 37-46. |
| [6] | LI Xiyan, ZHANG Hong, LIU Xuejing, YANG Hao, XU Shuai, LI Jiaxin, XIE Jiaqi, XU Guangwen. Study on decomposition characteristic and kinetics of magnesite in inhibitory atmosphere [J]. Inorganic Chemicals Industry, 2023, 55(10): 50-55. |
| [7] | WU Di, LI Laishi, WANG Junkai, WU Yusheng, WANG Yuzheng, LI Mingchun. Study on decomposition process and thermal decomposition kinetics of ammonium sulfate [J]. Inorganic Chemicals Industry, 2023, 55(10): 86-92. |
| [8] | ZHU Yue,QIU Shengbo,LIU Chenglin,YU Jianguo. Study on enhancement of spodumene phase reconstruction process by mechanical activation [J]. Inorganic Chemicals Industry, 2023, 55(1): 81-86. |
| [9] | ZHANG Shaokang,ZHAO Hua,LIU Runjing. Study on macro?kinetics of carbonization reaction of ammonia alkali salt sludge [J]. Inorganic Chemicals Industry, 2022, 54(6): 120-124. |
| [10] | ZHANG Shaokang,ZHAO Hua,LIU Runjing. Study on macro?kinetics of carbonization reaction of ammonia alkali salt sludge [J]. Inorganic Chemicals Industry, 2022, 54(6): 120-124. |
| [11] | YAO Haiwei,MAO Rui,WANG Fei,ZHU Zuoqiao. Study on high-value utilization of zinc oxide powder in rotary hearth furnace [J]. Inorganic Chemicals Industry, 2022, 54(12): 119-125. |
| [12] | DENG Liangming,JIANG Zhishui,LI Yihuang. Experimental study on compound refining of salt lake bischofite [J]. Inorganic Chemicals Industry, 2022, 54(10): 52-56. |
| [13] | ZHENG Hanxiao,LÜ Li,TANG Shengwei,HE Yanjun,ZHANG Tao. Leaching behavior of phosphate ore in phosphoric acid [J]. Inorganic Chemicals Industry, 2022, 54(10): 96-101. |
| [14] | ZHOU Jiaqi,CHEN Kui,WU Bin,JI Lijun,WU Yanyang. Study on kinetics of phosphate tailings calcination and acid leaching process [J]. Inorganic Chemicals Industry, 2022, 54(1): 77-82. |
| [15] | CAO Xuechen,ZHOU Dongjie,YAO Haiwei,JU Dianchun. Study on preparation of nanometer iron oxide and its kinetics by CC-OA DES treatment of iron-containing dust [J]. Inorganic Chemicals Industry, 2021, 53(11): 100-106. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||