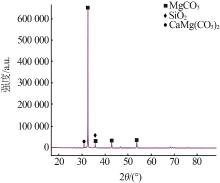
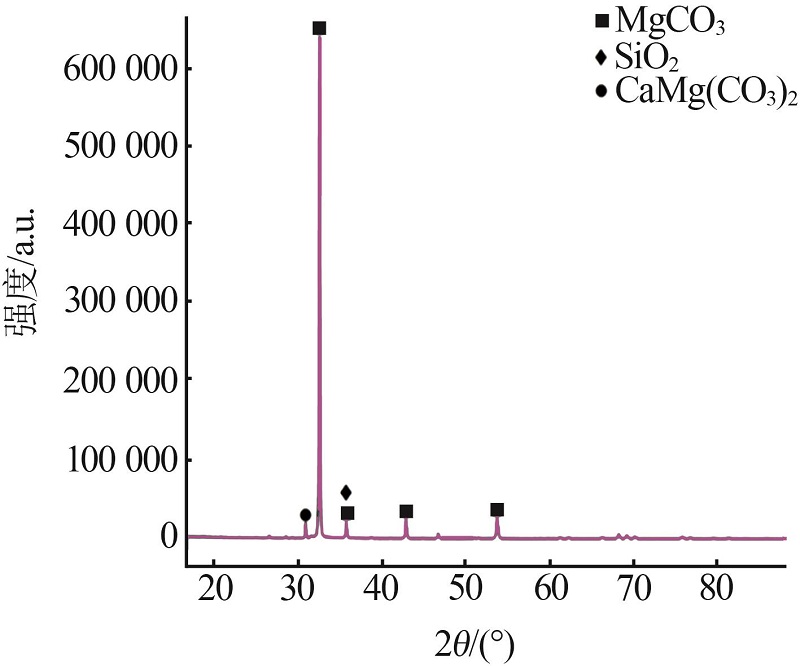
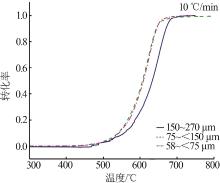
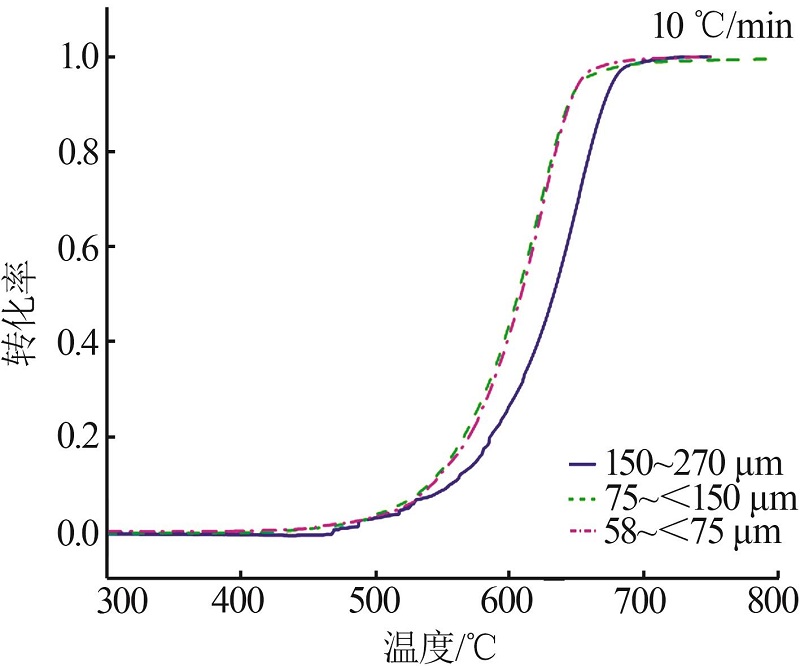
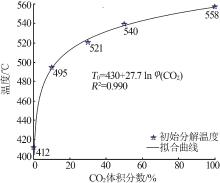
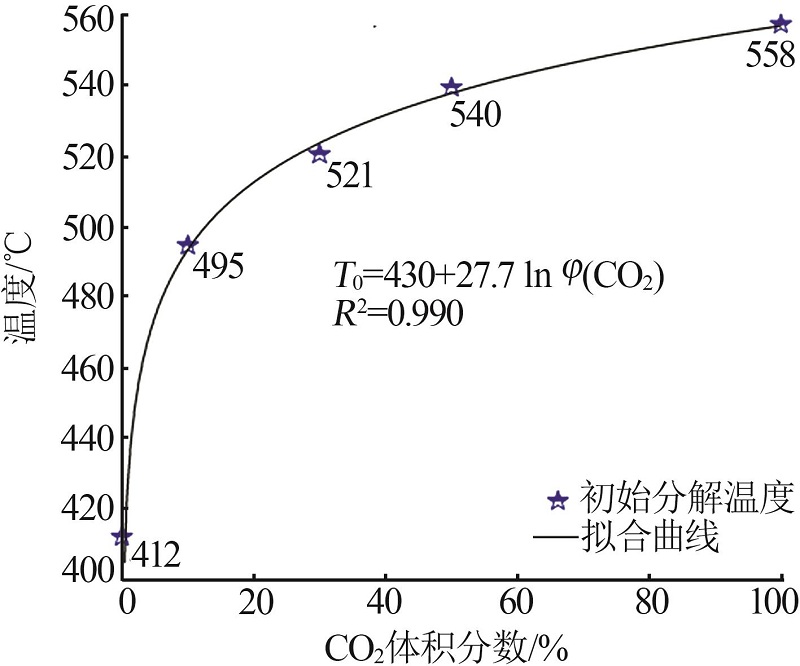
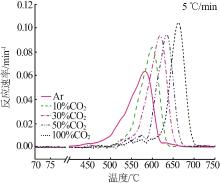
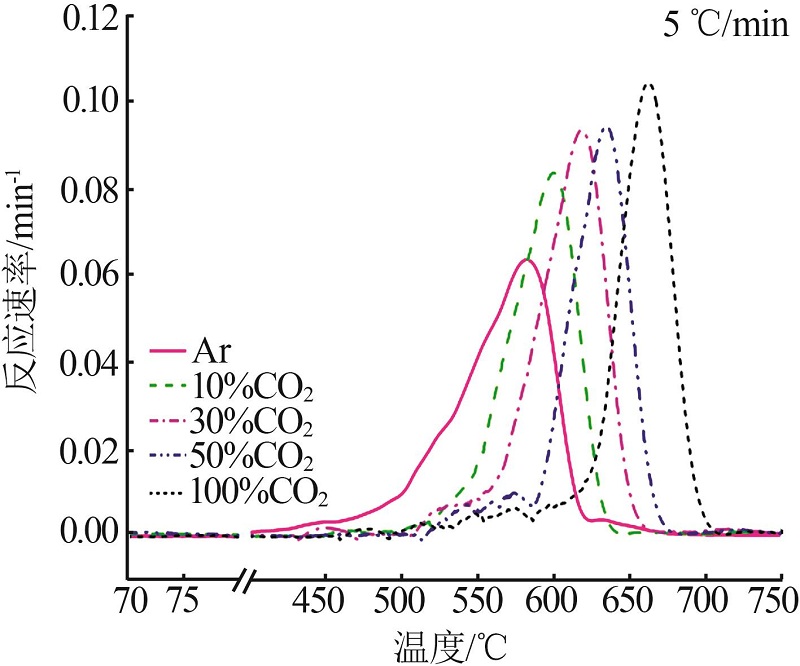
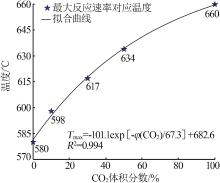
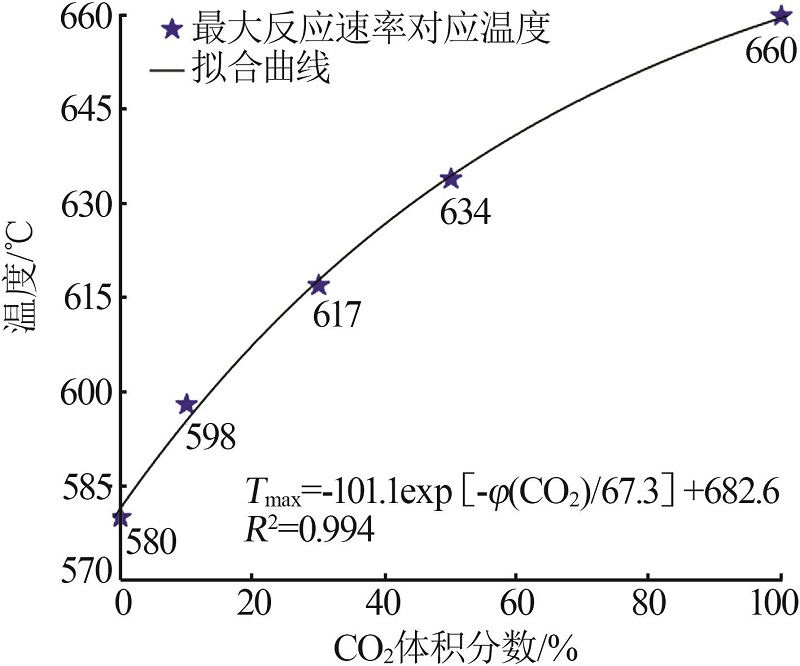
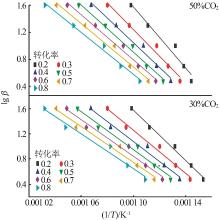
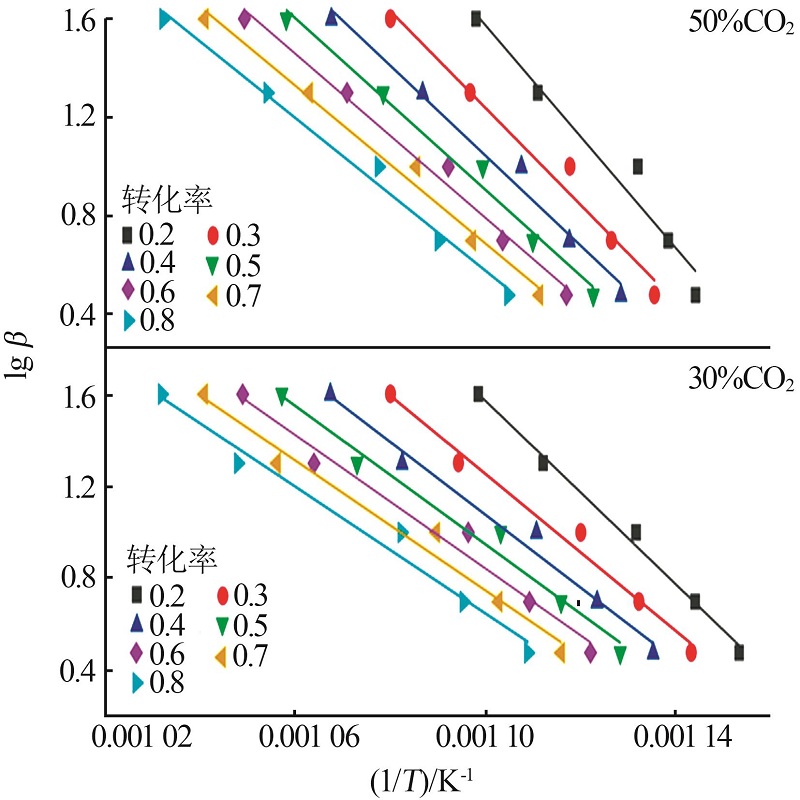
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (10): 50-55.doi: 10.19964/j.issn.1006-4990.2022-0696
• Research & Development • Previous Articles Next Articles
Study on decomposition characteristic and kinetics of magnesite in inhibitory atmosphere
LI Xiyan( ), ZHANG Hong, LIU Xuejing(
), ZHANG Hong, LIU Xuejing( ), YANG Hao, XU Shuai, LI Jiaxin, XIE Jiaqi, XU Guangwen
), YANG Hao, XU Shuai, LI Jiaxin, XIE Jiaqi, XU Guangwen
- Key Laboratory on Resources Chemicals and Material of Ministry of Education,ShenyangUniversity of Chemical Technology,Shenyang 110142,China
-
Received:2022-11-24Online:2023-10-10Published:2023-10-16 -
Contact:LIU Xuejing E-mail:1378790487@qq.com;liuxuejing6@163.com
CLC Number:
Cite this article
LI Xiyan, ZHANG Hong, LIU Xuejing, YANG Hao, XU Shuai, LI Jiaxin, XIE Jiaqi, XU Guangwen. Study on decomposition characteristic and kinetics of magnesite in inhibitory atmosphere[J]. Inorganic Chemicals Industry, 2023, 55(10): 50-55.
share this article
Table 2
Fitting results using Coats-Redfern formula for literature-reported mechanism function based on TGA data"
| 气氛 | G(x) | β→0 | β=5 ℃/min | β=10 ℃/min | β=20 ℃/min | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
E/ (kJ∙mol-1) | E/ (kJ∙mol-1) | R | E/ (kJ∙mol-1) | R | E/ (kJ∙mol-1) | R | |||||
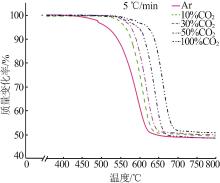
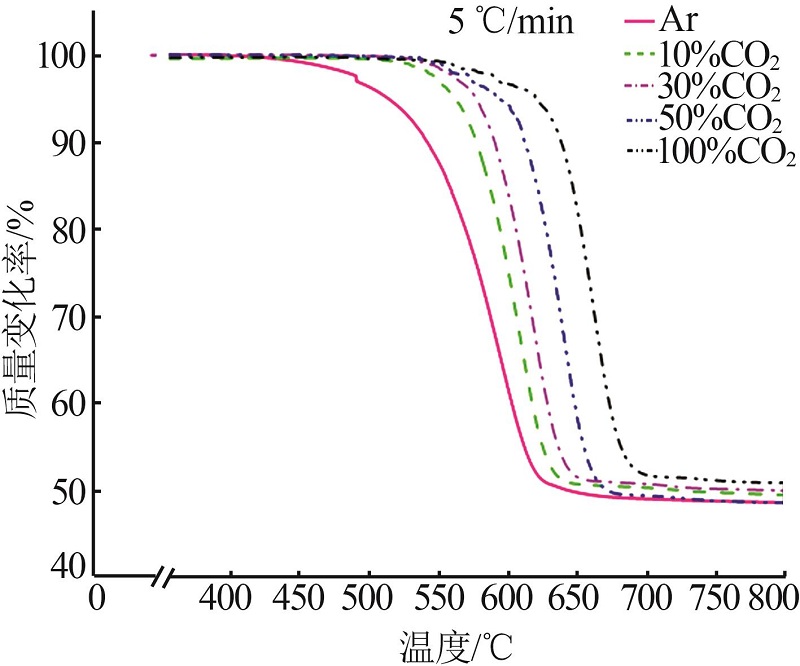
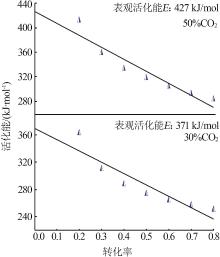
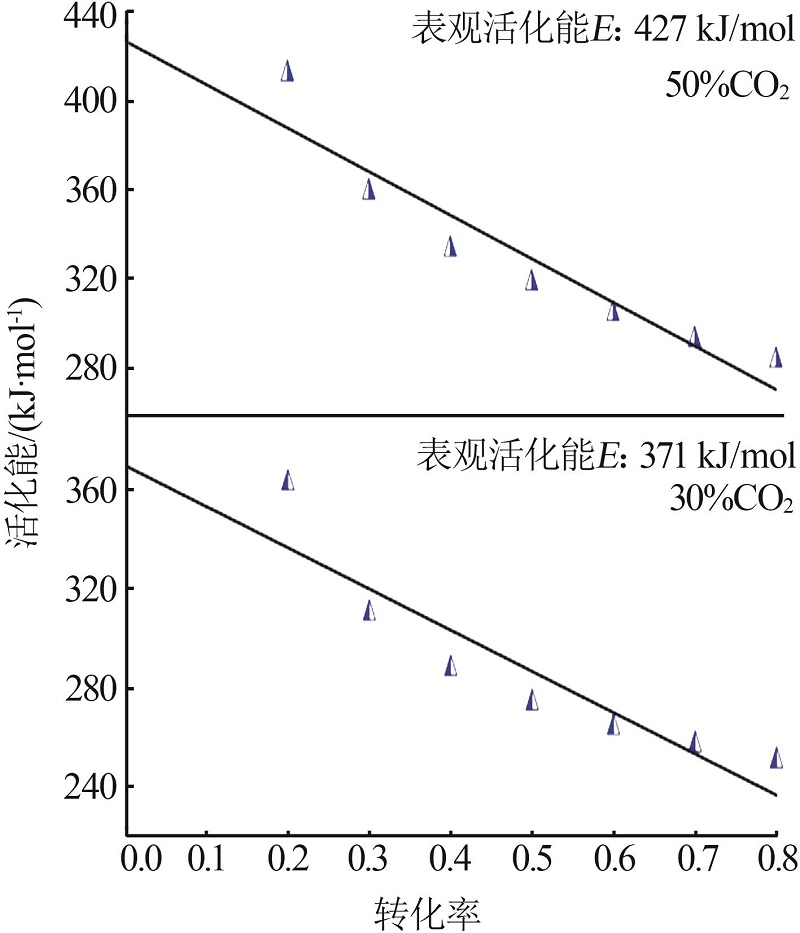
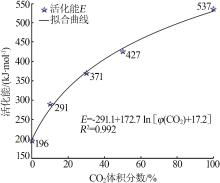
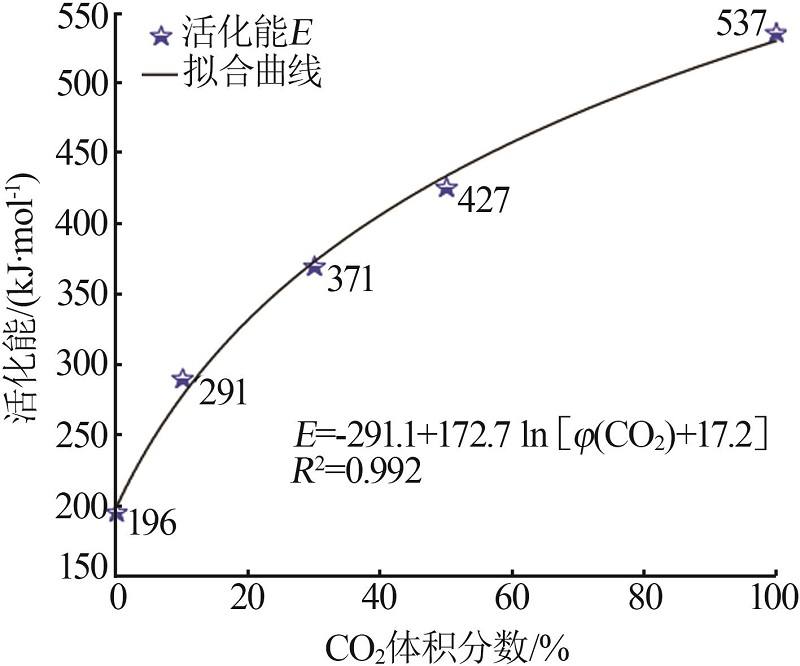
| 30%CO2 | G(x)=-ln(1-x) | 344 | 324 | 0.999 | 318 | 0.999 | 241 | 0.996 | |||
| G(x)=1-(1-x)1/2 | 258 | 287 | 0.996 | 265 | 0.999 | 201 | 0.999 | ||||
| G(x)=1-(1-x)1/3 | 275 | 305 | 0.998 | 282 | 0.999 | 214 | 0.999 | ||||
| 50%CO2 | G(x)=-ln(1-x) | 361 | 403 | 0.999 | 326 | 0.998 | 289 | 0.999 | |||
| G(x)=1-(1-x)1/2 | 301 | 273 | 0.998 | 241 | 0.999 | 231 | 0.995 | ||||
| G(x)=1-(1-x)1/3 | 320 | 290 | 0.999 | 256 | 0.999 | 245 | 0.997 | ||||
| 1 | 宋闯.菱镁矿尾矿处理与再利用研究进展[J].环境保护与循环经济,2022,42(3):12-15. |
| SONG Chuang.Research progress in the treatment and reuse of magnesite tailings[J].Environmental Protection and Circular Economy,2022,42(3):12-15. | |
| 2 | 丁怡,李军生,王风,等.中国菱镁产业发展现状及趋势[J].佛山陶瓷,2021,31(11):1-5,12. |
| DING Yi, LI Junsheng, WANG Feng,et al.Current situation and trend of Chinese magnesite industry[J].Foshan Ceramics,2021,31(11):1-5,12. | |
| 3 | 彭强,郭玉香,曲殿利.菱镁矿热分解的动力学研究[J].硅酸盐通报,2017,36(6):1886-1890. |
| PENG Qiang, GUO Yuxiang, QU Dianli.Thermal decomposition kinetics analysis of magnesite[J].Bulletin of the Chinese Ceramic Society,2017,36(6):1886-1890. | |
| 4 | ZHAO Yuna, ZHU Guocai.Thermal decomposition kinetics and mechanism of magnesium bicarbonate aqueous solution[J].Hydrometallurgy,2007,89(3/4):217-223. |
| 5 | 鲁志燕,韩露,吴锋,等.新疆尖山菱镁矿一步煅烧制备烧结镁砂[J].耐火材料,2022,56(2):158-161. |
| LU Zhiyan, HAN Lu, WU Feng,et al.Preparation of sintered magnesia by one-step calcination from Xinjiang Jianshan magnesite[J].Refractories,2022,56(2):158-161. | |
| 6 | 陈建铭,牛晓红,王晓彤,等.采用定-转子碳化反应器制备高活性氧化镁[J].无机盐工业,2016,48(12):40-43. |
| CHEN Jianming, NIU Xiaohong, WANG Xiaotong,et al.Preparation of highly active magnesium oxide by rotor-stator reactor(RSR)[J].Inorganic Chemicals Industry,2016,48(12):40-43. | |
| 7 | 李会东,杨莉萍,陶冶,等.玻璃纤维可燃物含量的热重分析方法研究[J].玻璃纤维,2022(4):36-42. |
| LI Huidong, YANG Liping, TAO Ye,et al.Research on thermogravimetric analysis for combustible content in glass fiber[J].Fiber Glass,2022(4):36-42. | |
| 8 | TIAN Lu, TAHMASEBI A, YU Jianglong.An experimental study on thermal decomposition behavior of magnesite[J].Journal of Thermal Analysis and Calorimetry,2014,118(3):1577-1584. |
| 9 | 白丽梅,邓玉芬,韩跃新,等.微细菱镁矿热分解过程及动力学[J].东北大学学报(自然科学版),2018,39(3):398-403. |
| BAI Limei, DENG Yufen, HAN Yuexin,et al.Thermal decomposition process and kinetics of microfine magnesite[J].Journal of Northeastern University(Natural Science),2018,39(3):398- 403. | |
| 10 | 王樱澈,刘雪景,许光文.含CO2抑制性气氛中菱镁矿分解反应动力学研究[J].辽宁化工,2020,49(12):1475-1478. |
| WANG Yingche, LIU Xuejing, XU Guangwen.Kinetics of decomposition of magnesite in inhibitory atmosphere containing CO2 [J].Liaoning Chemical Industry,2020,49(12):1475-1478. | |
| 11 | LIU Xuejing, HAO Wenqian, WANG Kexin,et al.Acquiring real kinetics of reactions in the inhibitory atmosphere containing product gases using micro fluidized bed[J].AIChE Journal,2021,67(9):17325. |
| 12 | YOUSEFZAD FARROKHI F, KAZANÇ F.Combustion behavior and kinetics of Turkish lignite blended with biomass/magnesite dust[J].Journal of Energy Engineering,2018,144(6):04018064. |
| 13 | 曾玺,王芳,余剑,等.微型流化床反应分析的方法基础与应用研究[J].化工进展,2016,35(6):1687-1697. |
| ZENG Xi, WANG Fang, YU Jian,et al.Fundamentals and applications of micro fluidized bed reaction analysis[J].Chemical Industry and Engineering Progress,2016,35(6):1687-1697. | |
| 14 | 任伟康,刘百宽,田晓利.新疆和静菱镁矿热分解特性及轻烧工艺研究[J].硅酸盐通报,2016,35(11):3556-3561,3568. |
| REN Weikang, LIU Baikuan, TIAN Xiaoli.Thermal decomposition and light burning process of Xinjiang Hejing magnesite[J].Bulletin of the Chinese Ceramic Society,2016,35(11):3556-3561,3568. | |
| 15 | 刘欣伟,冯雅丽,李浩然.菱镁矿热分解微分方程的建立[J].无机盐工业,2011,43(11):15-18. |
| LIU Xinwei, FENG Yali, LI Haoran.Establishment of differential equations of magnesite thermal decomposition[J].Inorganic Chemicals Industry,2011,43(11):15-18. | |
| 16 | TIAN L N, CHEN H P, YANG H P,et al.Study of limestone thermal decomposition in O2/CO2 atmosphere[C]∥International Symposium on Coal Combustion.Berlin,Heidelberg:Springer,2013:1283-1289. |
| 17 | 姜微微,郝文倩,刘雪景,等.微型流化床内菱镁矿轻烧反应特性及动力学[J].化工学报,2019,70(8):2928-2937. |
| JIANG Weiwei, HAO Wenqian, LIU Xuejing,et al.Characteristic and kinetics of light calcination of magnesite in micro fluidized bed reaction analyzer[J].CIESC Journal,2019,70(8):2928-2937. | |
| 18 | MAITRA S, MUKHERJEE S, SAHA N,et al.Non-isothermal decomposition kinetics of magnesite[J].Ceramic,2007,53(327):284-287. |
| [1] | GAN Shunpeng, DING Ding, SUN Chenggao, JIANG Shipeng, GUAN Junfang. Study on cold decomposition crystallization process of underground primary high-sodium carnallite ore [J]. Inorganic Chemicals Industry, 2024, 56(1): 67-72. |
| [2] | ZHANG Lei, LI Meng, XIANG Wenguo, HU Jun, CHEN Shiyi, DUAN Lunbo. Feasibility analysis of calcination and decomposition process of phosphogypsum in circulating fluidized bed [J]. Inorganic Chemicals Industry, 2023, 55(6): 85-91. |
| [3] | ZHANG Lingfeng, FAN Yajuan, MAO Chenchen, WU Shiguo, GU Hongxia. Research progress of cocatalyst of nicke-based catalyst system for hydrogen production from ammonia decomposition [J]. Inorganic Chemicals Industry, 2023, 55(3): 21-27. |
| [4] | ZHOU Qiang, WU Bin, CHEN Kui, JI Lijun, WU Yanyang. Study on thermal decomposition kinetic mechanism and calcination process of phosphorus tailings [J]. Inorganic Chemicals Industry, 2023, 55(3): 47-54. |
| [5] | TIAN Xiaoli, LI Zhixun, FENG Runtang, ZHANG Jie, ZHENG Quanfu, SHI Xuwu, DU Yongbin. Study on thermal decomposition behavior of Tibetan Kamado microcrystalline magnesite [J]. Inorganic Chemicals Industry, 2023, 55(3): 60-65. |
| [6] | ZHANG Xing,XU Jie,WANG Zibing,HOU Peng,HE Long,LIU Huan. Effect of feedstock particle size on kinetics of limestone thermal decomposition reaction [J]. Inorganic Chemicals Industry, 2023, 55(2): 79-84. |
| [7] | CHEN Xiaoqing, ZHOU Jian′an, WANG Yi, HAN Juan, WANG Bao, PEI Peiyan. Study on decomposition characteristic of limestone powder in high temperature flue gas of converter [J]. Inorganic Chemicals Industry, 2023, 55(10): 70-77. |
| [8] | WU Di, LI Laishi, WANG Junkai, WU Yusheng, WANG Yuzheng, LI Mingchun. Study on decomposition process and thermal decomposition kinetics of ammonium sulfate [J]. Inorganic Chemicals Industry, 2023, 55(10): 86-92. |
| [9] | TANG Jianping,WANG Guosheng. Study on preparation technology and waterproof property of fused magnesia coating [J]. Inorganic Chemicals Industry, 2022, 54(5): 90-95. |
| [10] | Huang Jiancui,Ling Guanshuang,Zong Jun. Study on new conditions for preparation of highly-dispersed hexagonal magnesium hydroxide nanoparticles from hydromagnesite [J]. Inorganic Chemicals Industry, 2021, 53(2): 55-60. |
| [11] | CHEN Xue,ZHANG Menghui,ZHAO Liang,DONG Hui,WANG Dexi. Study on pyrolysis process of Mg(NO3)2·nH2O [J]. Inorganic Chemicals Industry, 2021, 53(12): 91-94. |
| [12] | ZHOU Lan,LI Wang,LIAO Wenjun. Research status and analysis of effect of interfacial chemistry on electrochemical properties of LiNi0.5Mn1.5O4 cathode materials [J]. Inorganic Chemicals Industry, 2021, 53(11): 17-24. |
| [13] | Jiao Shuai,Yi Shouzhi,Zhang Hongling,Chen Huixia,Cheng Xichuan,Zhang Lichang,Xu Hongbin. Effect of reaction condition and aging process on the oxidation rate of chromium hydroxide [J]. Inorganic Chemicals Industry, 2021, 53(10): 59-63. |
| [14] | Yin Wei,Huang Jin,Xie Tian,He Bingbing. Experimental study on high efficiency decomposition of phosphate rock by phosphoric acid [J]. Inorganic Chemicals Industry, 2020, 52(9): 34-36. |
| [15] | Gong Jiazhu,Lu Xiangfang,Wu Ninglan,Shao Guoxiong. Research and development of self-fitting nano-sized titanium dioxide catalytic wastewater treatment agent [J]. Inorganic Chemicals Industry, 2020, 52(5): 59-64. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||