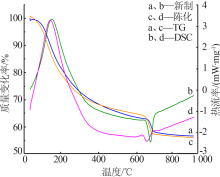
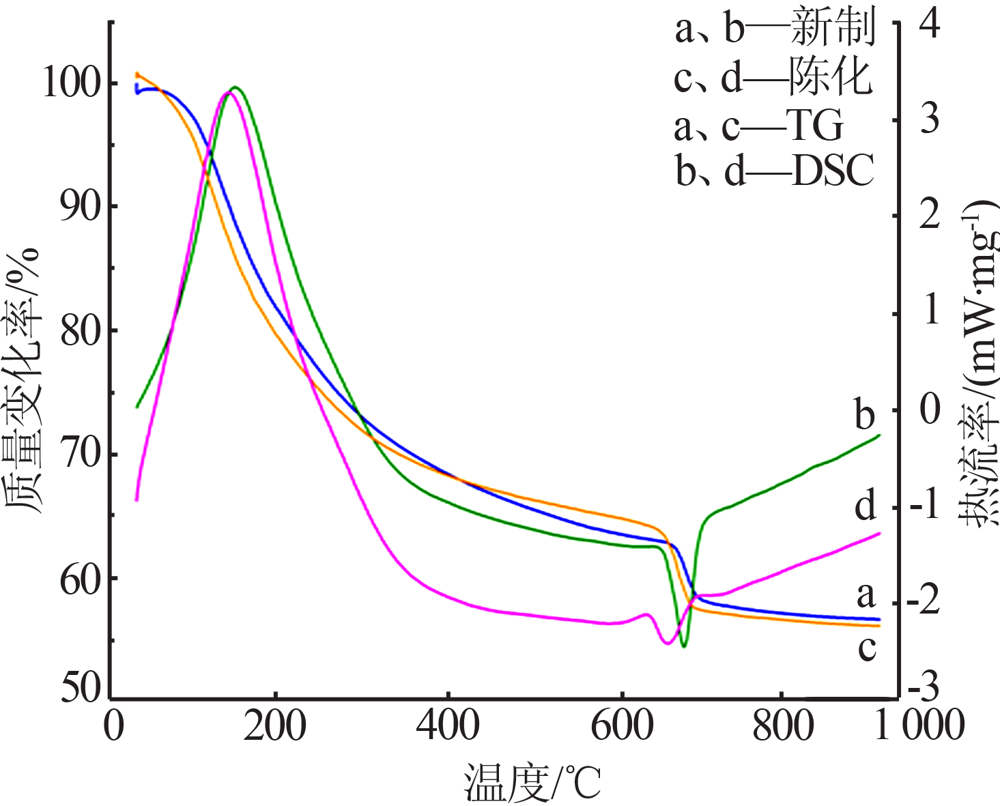
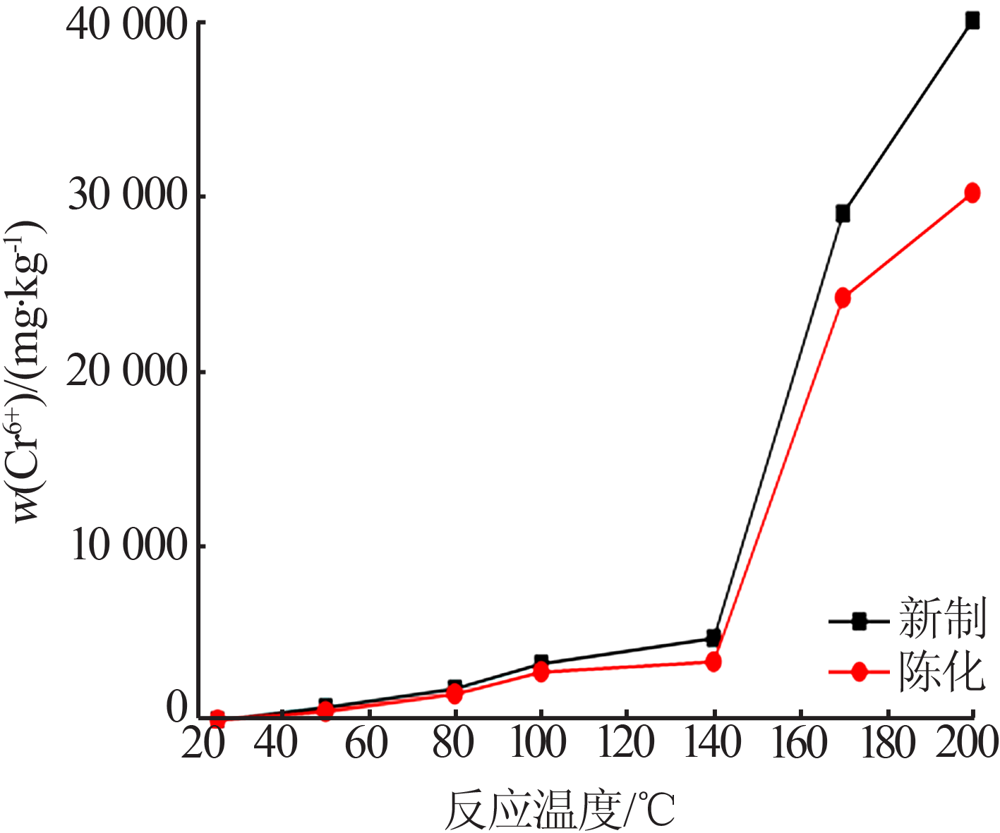
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (10): 59-63.doi: 10.19964/j.issn.1006-4990.2020-0695
• Research & Development • Previous Articles Next Articles
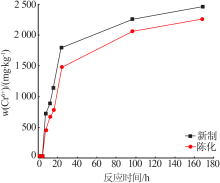
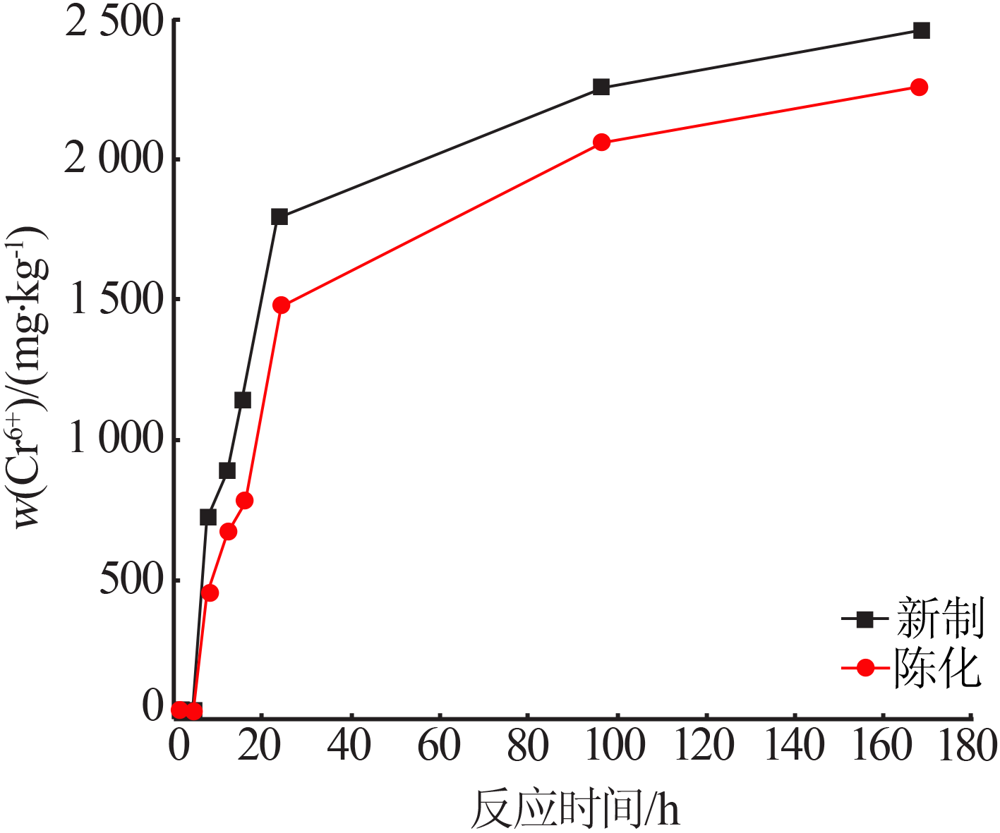
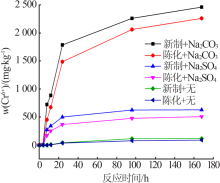
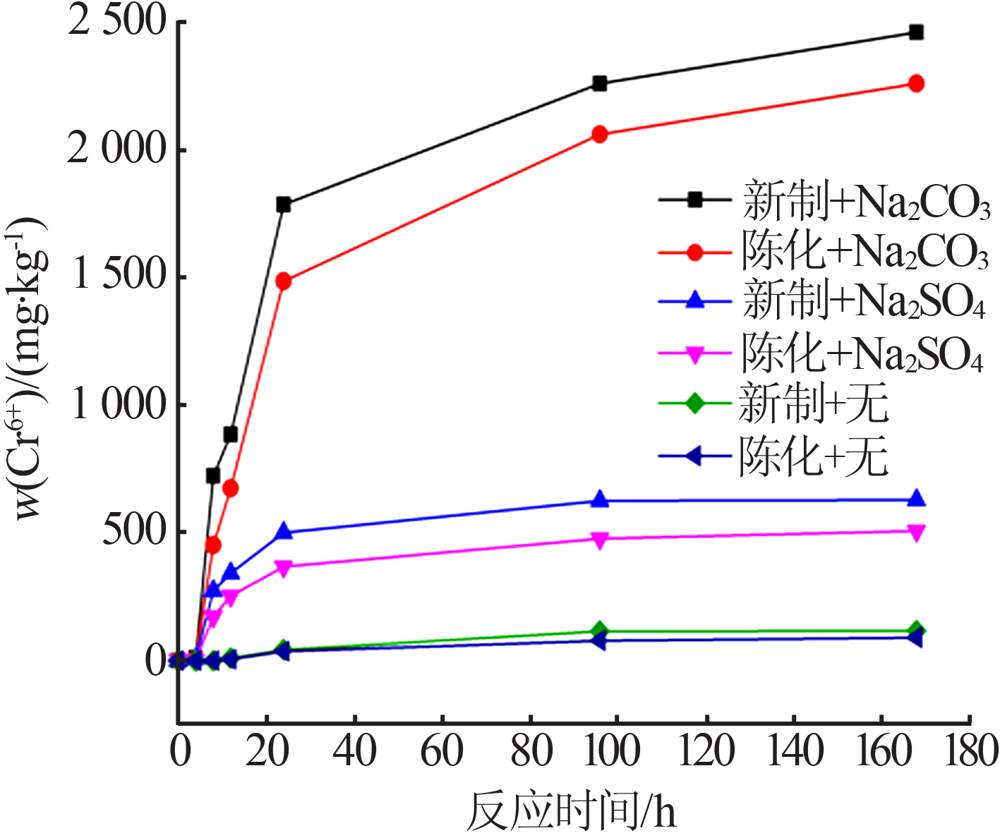
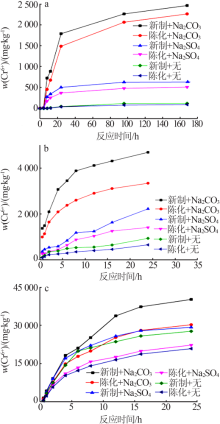
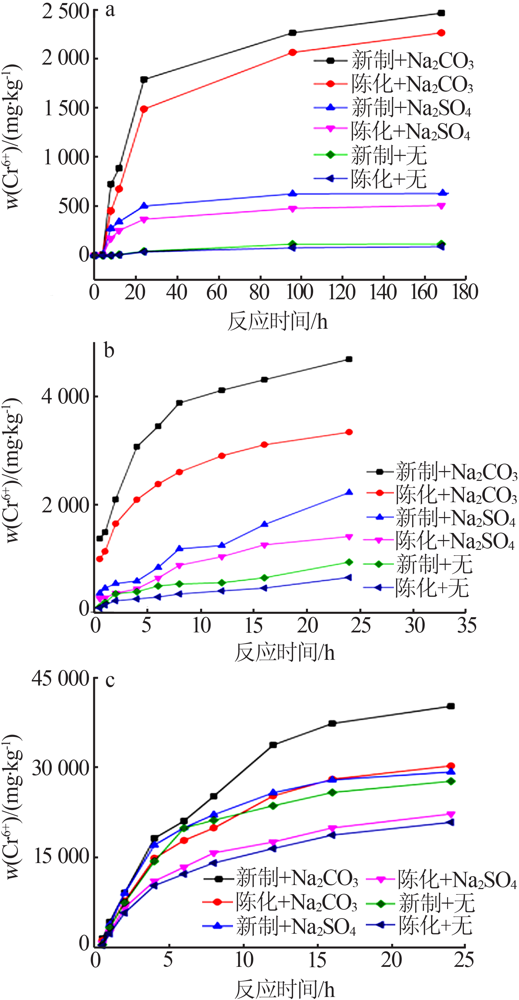
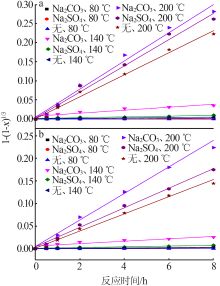
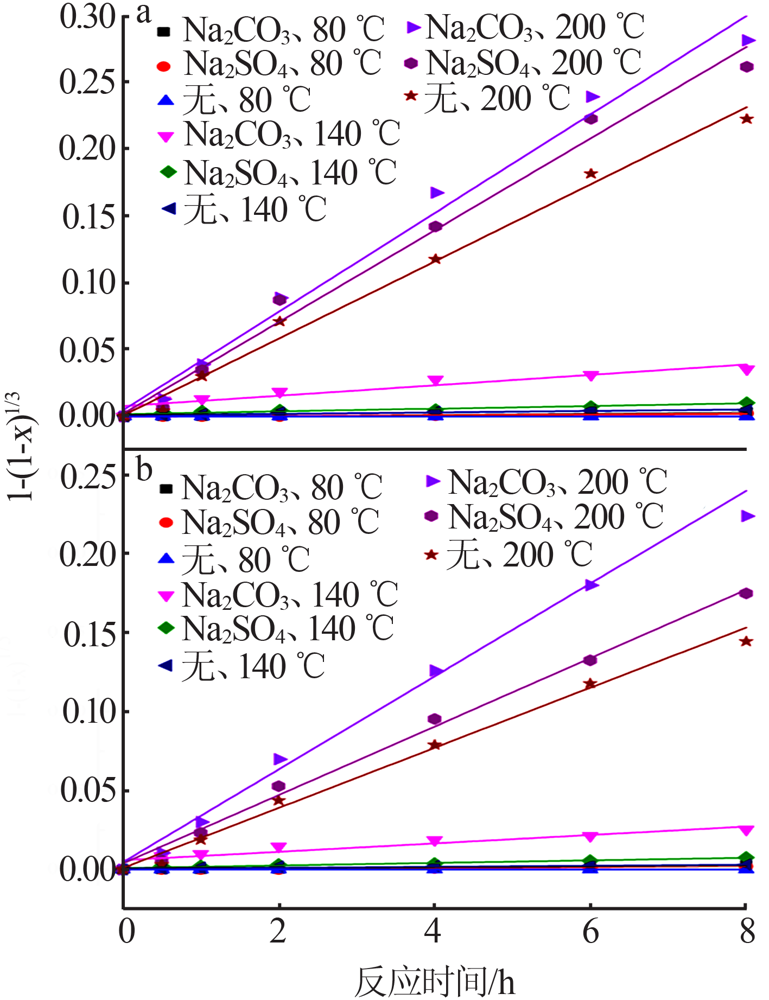
Effect of reaction condition and aging process on the oxidation rate of chromium hydroxide
Jiao Shuai1,2,3( ),Yi Shouzhi1(
),Yi Shouzhi1( ),Zhang Hongling2,3,4(
),Zhang Hongling2,3,4( ),Chen Huixia2,3,Cheng Xichuan5,Zhang Lichang6,Xu Hongbin2,3,4
),Chen Huixia2,3,Cheng Xichuan5,Zhang Lichang6,Xu Hongbin2,3,4
- 1. College of Chemical Engineering and Materials,Tianjin University of Science and Technology,Tianjin 300222,China
2. Key Laboratory of Green Process and Engineering,Institute of Process Engineering,Chinese Academy of Sciences
3. National Engineering Laboratory of Hydrometallurgy and Clean Production Technology,Institute of Process Engineering,Chinese Academy of Sciences
4. University of Chinese Academy of Sciences
5. Hubei Zhenhua Chemical Co.,Ltd.
6. Yuhuan Environmental Technology Co.,Ltd.