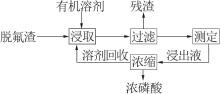
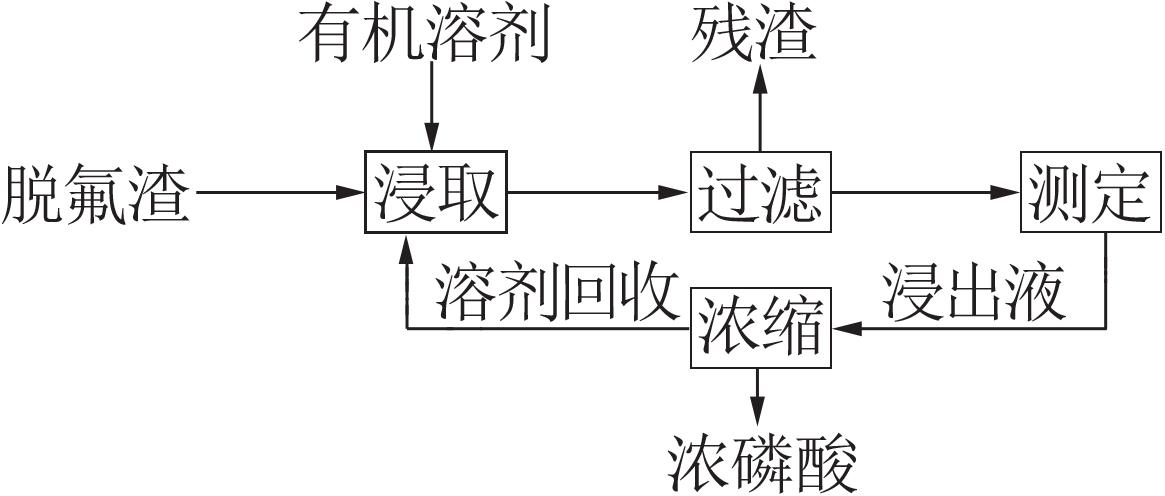
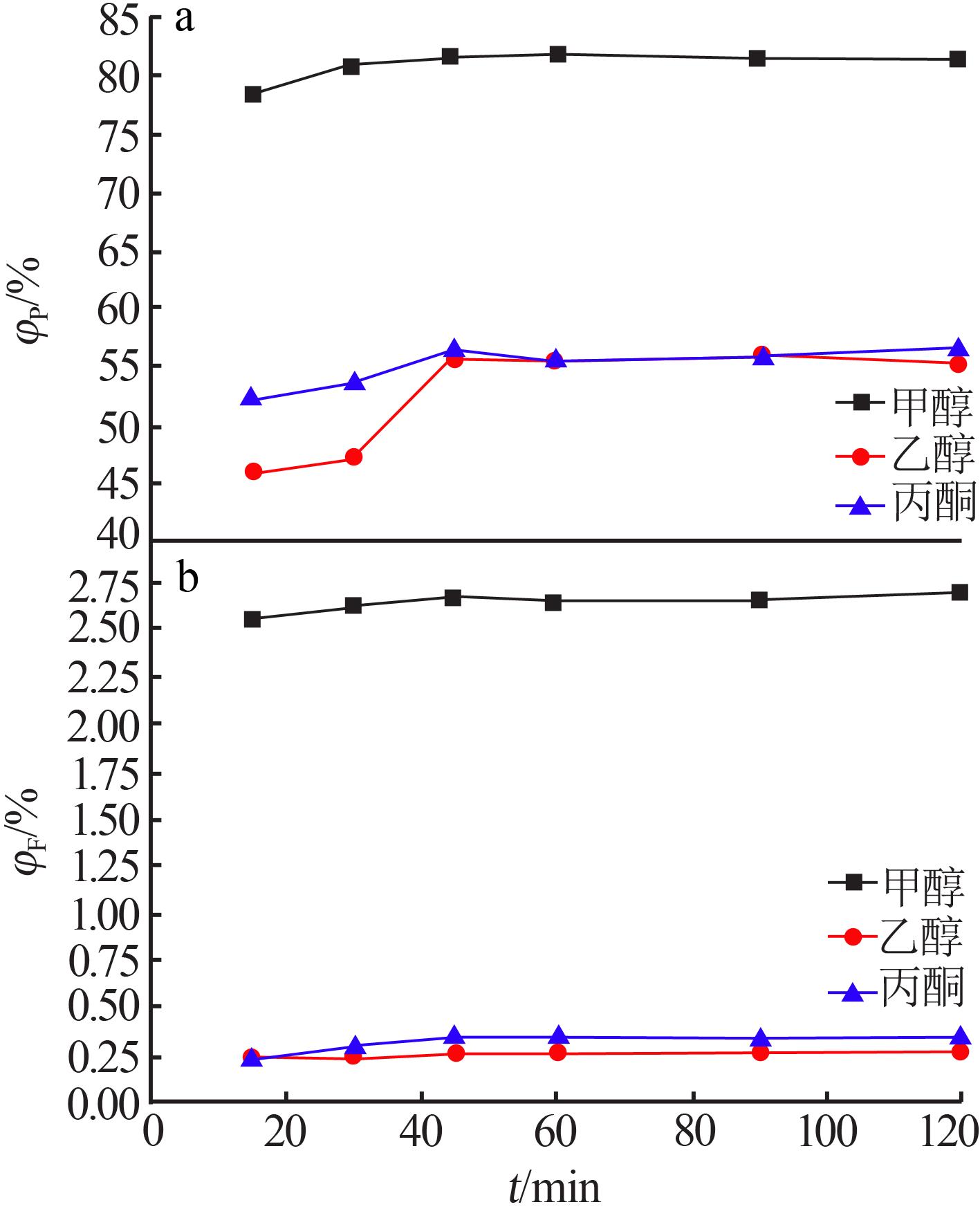
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (7): 129-134.doi: 10.19964/j.issn.1006-4990.2021-0654
• Environment·Health·Safety • Previous Articles Next Articles
Study on preparation of phosphoric acid by leaching fluoride residue from wet-process phosphoric acid with organic solvents
FU Ziqi1( ), ZHANG Cheng1, SHENG Yong2, JI Lijun1(
), ZHANG Cheng1, SHENG Yong2, JI Lijun1( )
)
- 1.School of Chemical Engineering,East China University of Science and Technology,Shanghai 200237,China
2.Sinochem Yunlong Co. ,Ltd.
-
Received:2021-10-27Online:2022-07-10Published:2022-07-14 -
Contact:JI Lijun E-mail:17621965230@163.com;jilijun@ecust.edu.cn
CLC Number:
Cite this article
FU Ziqi, ZHANG Cheng, SHENG Yong, JI Lijun. Study on preparation of phosphoric acid by leaching fluoride residue from wet-process phosphoric acid with organic solvents[J]. Inorganic Chemicals Industry, 2022, 54(7): 129-134.
share this article
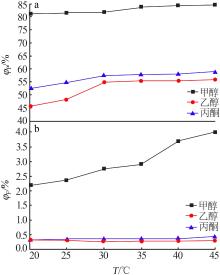
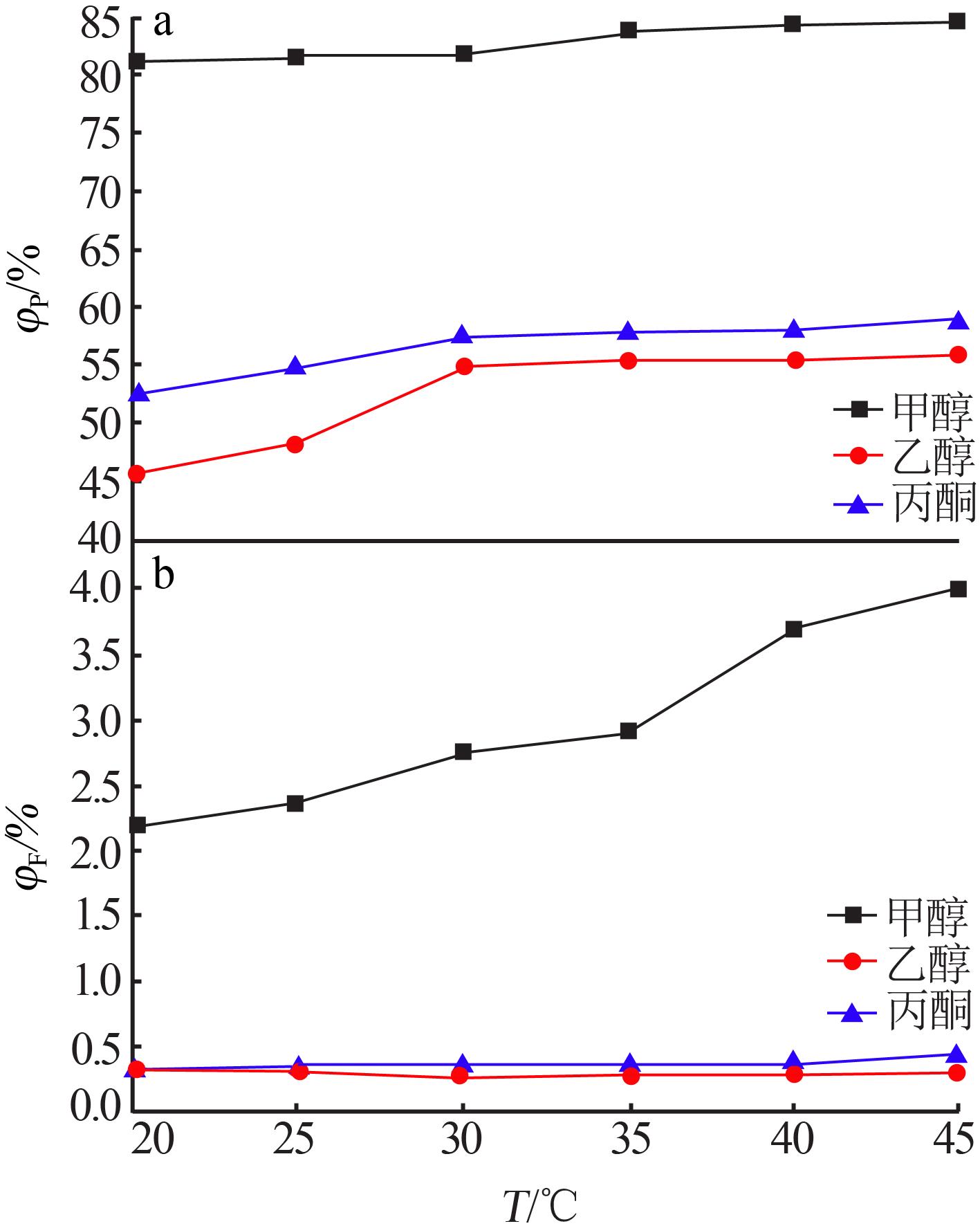
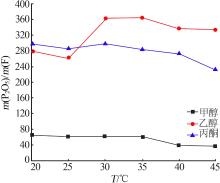
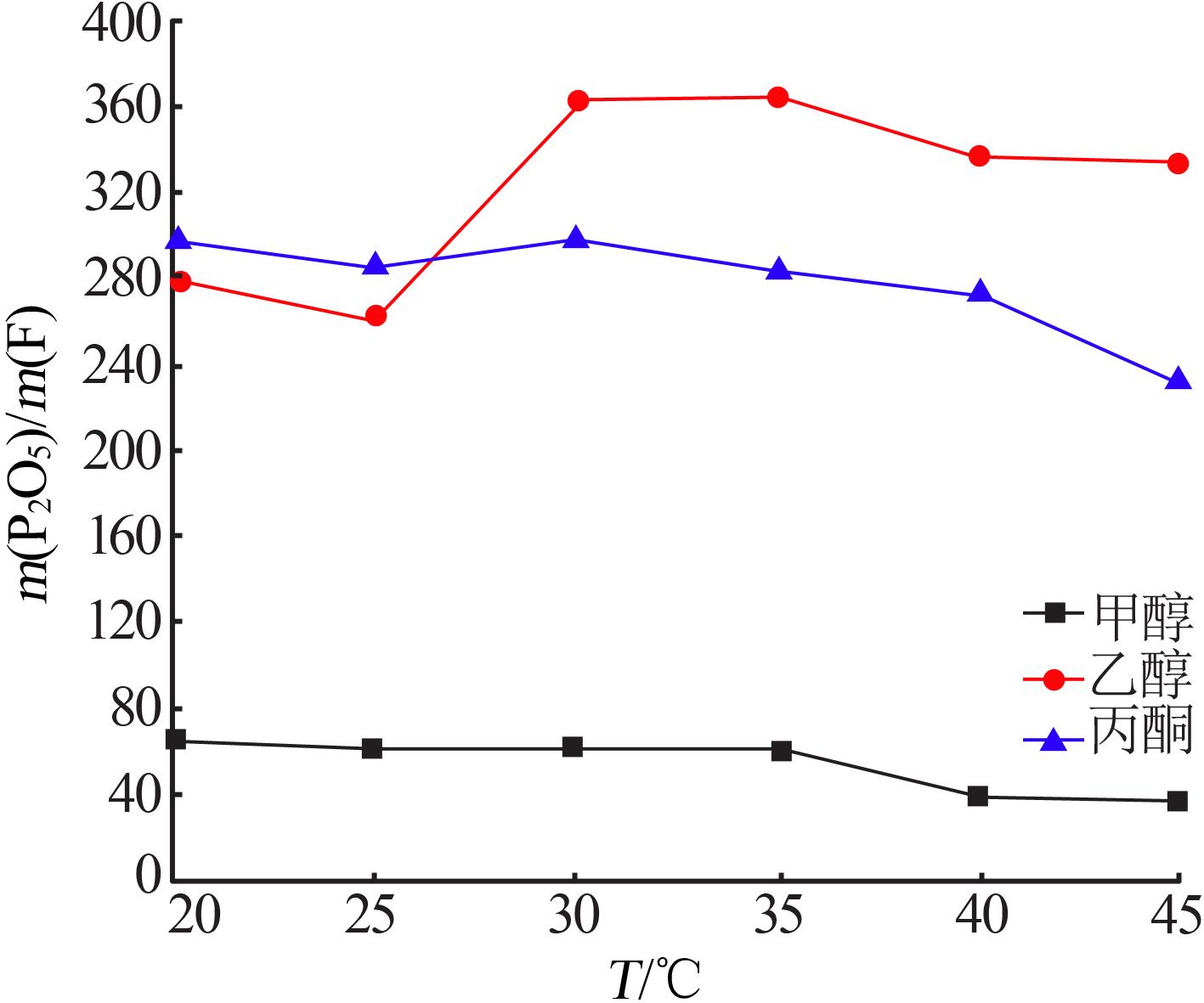
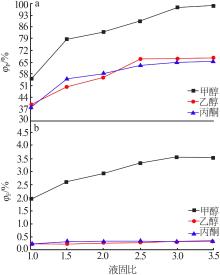
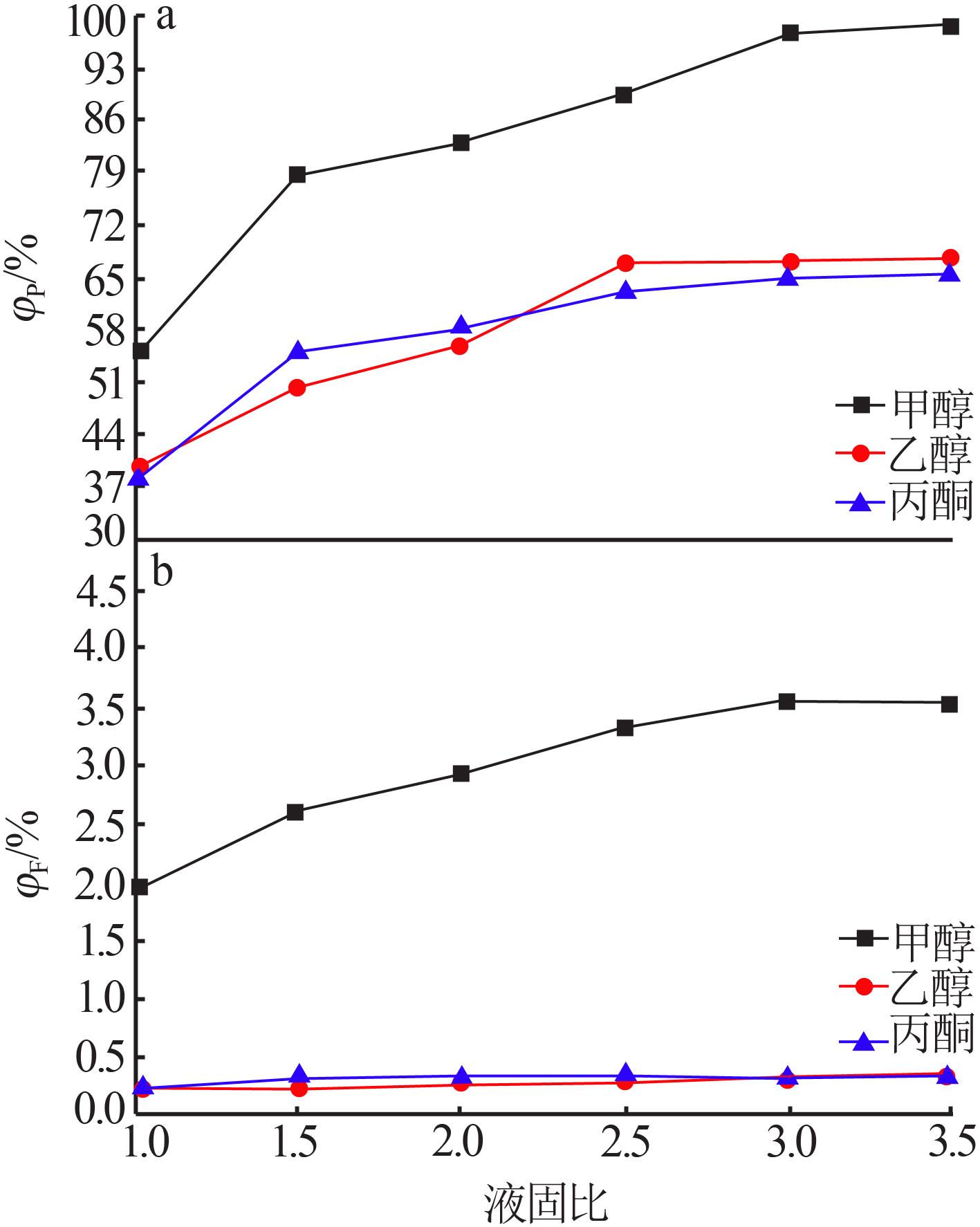
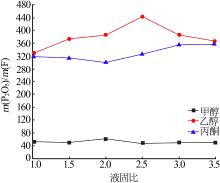
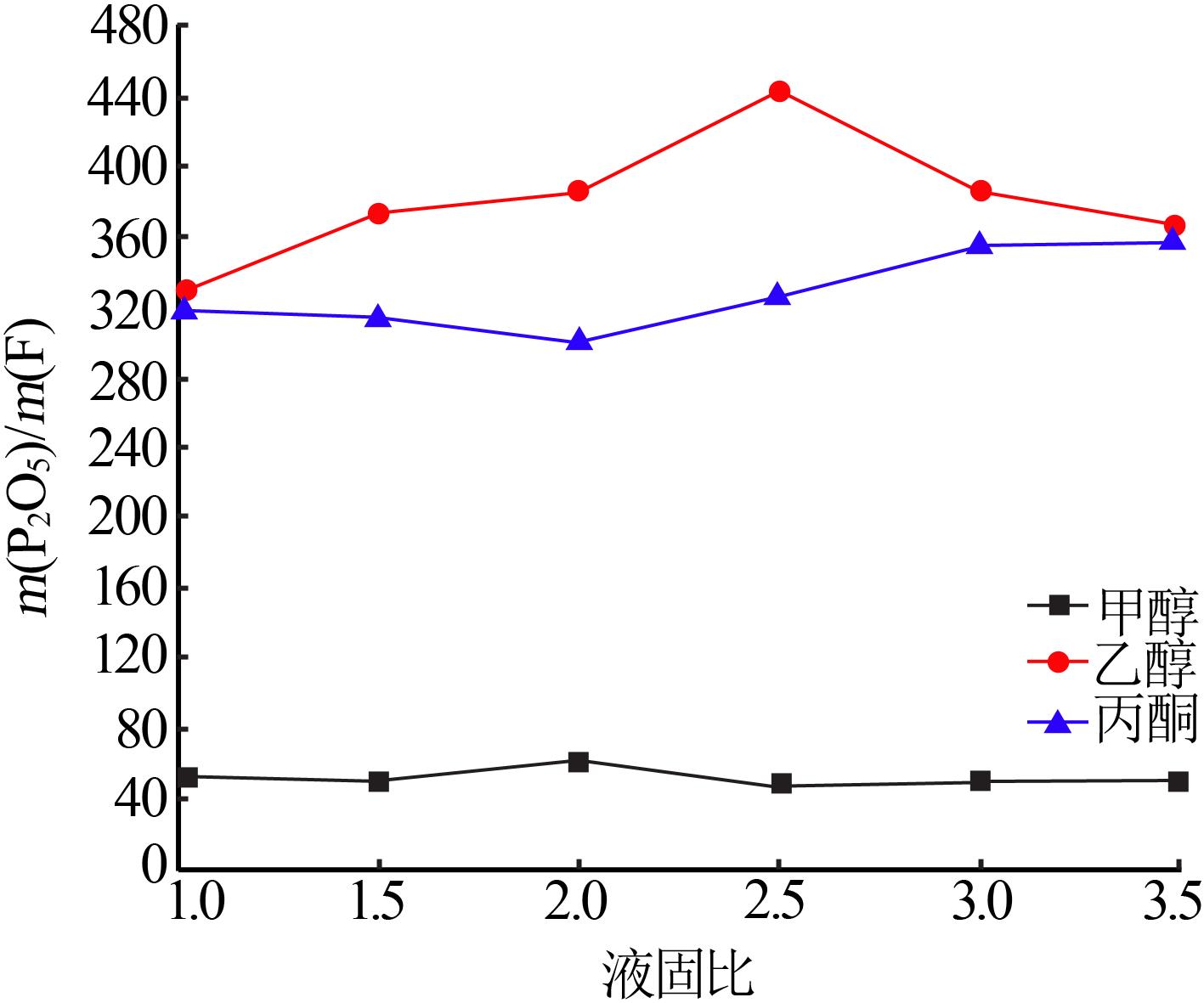
Table 4
Results of response surface test"
| 序号 | A | B | C | m(P2O5)/ m(F) | |
|---|---|---|---|---|---|
| 1 | -1 | 0 | 1 | 90.57 | 48.03 |
| 2 | -1 | 1 | 0 | 86.93 | 51.22 |
| 3 | -1 | -1 | 0 | 84.32 | 50.13 |
| 4 | -1 | 0 | -1 | 81.79 | 60.04 |
| 5 | 0 | 0 | 0 | 89.91 | 49.11 |
| 6 | 0 | 0 | 0 | 89.33 | 48.23 |
| 7 | 0 | -1 | 1 | 96.20 | 49.36 |
| 8 | 0 | 0 | 0 | 89.52 | 48.02 |
| 9 | 0 | 0 | 0 | 89.58 | 49.25 |
| 10 | 0 | 1 | -1 | 82.03 | 62.53 |
| 11 | 0 | 1 | 1 | 97.76 | 49.45 |
| 12 | 0 | -1 | -1 | 80.80 | 60.36 |
| 13 | 0 | 0 | 0 | 89.65 | 49.03 |
| 14 | 1 | -1 | 0 | 94.97 | 32.87 |
| 15 | 1 | 0 | 1 | 98.55 | 34.62 |
| 16 | 1 | 1 | 0 | 96.05 | 35.38 |
| 17 | 1 | 0 | -1 | 84.31 | 40.05 |
Table 5
Regression model analysis of variance"
| 变量 | 自由度 | 平方和 | 均方 | F值 | p值 | 显著性 |
|---|---|---|---|---|---|---|
| A | 1 | 544.83 | 544.83 | 525.31 | <0.000 1 | ** |
| B | 1 | 5.02 | 5.02 | 4.84 | 0.063 6 | |
| C | 1 | 215.49 | 215.49 | 207.77 | <0.000 1 | ** |
| AB | 1 | 0.902 5 | 0.902 5 | 0.870 2 | 0.382 0 | |
| AC | 1 | 10.82 | 10.82 | 10.44 | 0.014 4 | * |
| BC | 1 | 1.08 | 1.08 | 1.04 | 0.341 2 | |
| A2 | 1 | 267.72 | 267.72 | 258.13 | <0.000 1 | ** |
| B2 | 1 | 13.13 | 13.13 | 12.66 | 0.009 2 | ** |
| C2 | 1 | 102.38 | 102.38 | 98.71 | <0.000 1 | ** |
| 模型 | 9 | 1 142.6 | 126.96 | 122.41 | <0.000 1 | ** |
| 残差 | 7 | 7.26 | 1.04 | |||
| 失拟项 | 3 | 6.00 | 2.00 | 6.36 | 0.053 0 | |
| 纯误差 | 4 | 1.26 | 0.314 7 | |||
| 总离差 | 16 | 1 149.87 |
| 1 | 李维,高辉,罗英杰,等.国内外磷矿资源利用现状、趋势分析及对策建议[J].中国矿业,2015,24(6):6-10. |
| LI Wei, GAO Hui, LUO Yingjie,et al.Status,trends and suggestions of phosphorus ore resources at home and abroad[J].China Mining Magazine,2015,24(6):6-10. | |
| 2 | 谢晓霞,陈春光,徐建,等.我国湿法磷酸工艺路线发展趋势[J].当代化工,2014,43(11):2350-2354. |
| XIE Xiaoxia, CHEN Chunguang, XU Jian,et al.Development trend of the wet phosphoric acid production process in China[J].Contemporary Chemical Industry,2014,43(11):2350-2354. | |
| 3 | 杨雄俊,周鑫,谭顺仓,等.湿法磷酸脱氟技术研究[J].磷肥与复肥,2017,32(8):8-9. |
| YANG Xiongjun, ZHOU Xin, TAN Shuncang,et al.Study on technology of fluoride removal from wet-process phosphoric acid[J].Phosphate & Compound Fertilizer,2017,32(8):8-9. | |
| 4 | 张团慧,向鹏,李国斌,等.湿法磷酸净化技术的研究进展[J].材料导报,2015,29(15):113-117. |
| ZHANG Tuanhui, XIANG Peng, LI Guobin,et al.Researches of purification technology for wet-process phosphoric acid[J].Materials Review,2015,29(15):113-117. | |
| 5 | AMIN M I, ALI M M, KAMAL H M,et al.Recovery of high grade phosphoric acid from wet process acid by solvent extraction with aliphatic alcohols[J].Hydrometallurgy,2010,105(1/2):115-119. |
| 6 | JIN Yang, MA Yujing, WENG Yanling,et al.Solvent extraction of Fe3+ from the hydrochloric acid route phosphoric acid by D2EHPA in kerosene[J].Journal of Industrial and Engineering Chemistry,2014,20(5):3446-3452. |
| 7 | 周权宝,李步通,左林桂,等.湿法磷酸中金属离子净化技术研究进展[J].应用化工,2017,46(7):1417-1423. |
| ZHOU Quanbao, LI Butong, ZUO Lingui,et al.Research progress on purification technology of metal ions in wet process phosphoric acid[J].Applied Chemical Industry,2017,46(7):1417-1423. | |
| 8 | 李燕凤.生产饲料级磷酸氢钙用湿法磷酸净化技术探讨[J].硫磷设计与粉体工程,2020(4):22-26,6. |
| LI Yanfeng.Review of technology for purification of wet-process phosphoric acid for production of feed grade calcium hydrogen phosphate[J].Sulphur Phosphorus & Bulk Materials Handling Related Engineering,2020(4):22-26,6. | |
| 9 | ELLEUCH M B C, AMOR M B, POURCELLY G.Phosphoric acid purification by a membrane process:Electrodeionization on ion-exchange textiles[J].Separation and Purification Technology,2006,51(3):285-290. |
| 10 | 李自炜,周萌,吴宁兰,等.全球饲料磷酸盐生产技术与发展趋势[J].无机盐工业,2016,48(4):6-12. |
| LI Ziwei, ZHOU Meng, WU Ninglan,et al.Globe production technology and development trend of feed-grade phosphate[J].Inorganic Chemicals Industry,2016,48(4):6-12. | |
| 11 | 霍云飞,陈俊.二水物法、半水物法、半水和二水物再结晶法湿法磷酸工艺的应用比较[J].磷肥与复肥,2013,28(4):32-36. |
| HUO Yunfei, CHEN Jun.Comparison of application of dihydrate method,hemihydrate method,hemihydrate and dihydrate recrystallization method for WPA production[J].Phosphate & Compound Fertilizer,2013,28(4):32-36. | |
| 12 | 钟文婧,纪利俊,付全军,等.湿法磷酸脱氟渣回收技术研究[J].化学工程,2019,47(1):70-73,78. |
| ZHONG Wenjing, JI Lijun, FU Quanjun,et al.Study on recycling of the fluoride residue from wet-process phosphoric acid[J].Che- | |
| Engineering(China) mical,2019,47(1):70-73,78. | |
| 13 | 张程,钟文婧,纪利俊,等.湿法磷酸脱氟渣中磷和氟的浸取回收[J].化学工程,2019,47(8):1-5,21. |
| ZHANG Cheng, ZHONG Wenjing, JI Lijun,et al.Recovery of phosphorus and fluorine in defluorination sludge from wet-process phosphoric acid by leaching[J].Chemical Engineering(China),2019,47(8):1-5,21. | |
| 14 | 李莉,张赛,何强,等.响应面法在试验设计与优化中的应用[J].实验室研究与探索,2015,34(8):41-45. |
| LI Li, ZHANG Sai, HE Qiang,et al.Application of response surface methodology in experiment design and optimization[J].Research and Exploration in Laboratory,2015,34(8):41-45. | |
| 15 | 钟文婧.湿法磷酸脱氟渣回收工艺研究[D].上海:华东理工大学,2018. |
| ZHONG Wenjing.Study on recovery technology of fluoride residue from wet process phosphoric acid[D].Shanghai:East China University of Science and Technology,2018. |
| [1] | YU Zhou, HE Zhaoyi, TANG Liang, HE Sheng, XIAO Haixin, XIAO Yixun. Study on preparation and microscopic properties of typical sulfate solid waste composite cementitious materials [J]. Inorganic Chemicals Industry, 2024, 56(4): 90-97. |
| [2] | WANG Jianrui, ZHANG Song, ZHANG Jie. Study on new process for beneficiation phosphate of low-grade phosphate rock leaching via lactic acid [J]. Inorganic Chemicals Industry, 2024, 56(3): 56-63. |
| [3] | WANG Ruting, ZHAO Xiaorong, HUANG Xuquan, WANG Haojie, XUE Fei, CAI Jiawei. Research on preparation and early performance of mixed phase phosphogypsum-based cementing materials [J]. Inorganic Chemicals Industry, 2024, 56(3): 98-104. |
| [4] | HUANG Tao, HUANG Zili, XIAO Shuo, ZHENG Jiemiao, LIU Xiaofeng, WU Jilong. Experimental study on preparation of polyferric chloride from iron tailings acid leaching solution [J]. Inorganic Chemicals Industry, 2024, 56(2): 121-126. |
| [5] | ZHANG Li, ZHANG Dan, PAN Hongyan, DONG Yonggang, LI Wenfei, QIN Hong. Study on preparation of low ash activated carbon by phosphoric acid method [J]. Inorganic Chemicals Industry, 2024, 56(2): 95-103. |
| [6] | XIA Guiying, YANG Liuchun, YUAN Zhiye. Study on direct leaching of rare earth elements from phosphogypsum with sulfuric acid [J]. Inorganic Chemicals Industry, 2024, 56(1): 107-113. |
| [7] | XIANG Mengqi, MENG Hua, WANG Ye, MENG Xianzhang, BAI Yuhang, WANG Yujunyao, ZHANG Yidan. Study on kinetic of iron leaching from titanium gypsum and its cyclic acid leaching process [J]. Inorganic Chemicals Industry, 2024, 56(1): 114-120. |
| [8] | ZHANG Ying, LI Jun, JIN Yang, HUANG Meiying. Study on pyrolysis regeneration of activated carbon for Tributyl phosphate decolorization [J]. Inorganic Chemicals Industry, 2024, 56(1): 59-66. |
| [9] | ZHANG Conghua, YAN Wenbin, XIAO Jiajun, ZHAO Ke, PENG Shangquan, WEI Yuhong. Reductive leaching technology of manganese anode slag using tartaric acid as reducing agent optimized by RSM [J]. Inorganic Chemicals Industry, 2023, 55(9): 106-113. |
| [10] | LUO Wenbo, LI Heng, LÜ Jun, YANG Linguang, ZHAO Xingfan, LONG Xiao. Study on recovery of silicon and aluminum from industrial silicon slag [J]. Inorganic Chemicals Industry, 2023, 55(9): 94-99. |
| [11] | WANG Yingnan, SHENG Linlin, HUANG Juan, HUANG Zhanbin. Study on adsorption performance of lead from water by coal-fired slag [J]. Inorganic Chemicals Industry, 2023, 55(8): 109-115. |
| [12] | DONG Xinyu, WANG Haifeng, HE Yue, YANG Pan, WANG Song, YANG Chunyuan, WANG Qin, HUANG Bifang, WANG Jiawei. Exleaching toxicity analysis and harmless treatment of electrolytic manganese residue [J]. Inorganic Chemicals Industry, 2023, 55(5): 85-90. |
| [13] | ZHOU Qinglie, WANG Baoqi, ZHANG Zhiye, ZHANG Yinghu, WANG Jian, YANG Lin. Development of new process of removing metal cations from wet-process phosphoric acid by extraction [J]. Inorganic Chemicals Industry, 2023, 55(3): 84-91. |
| [14] | DONG Xiongwei, HAN Fenglan, HUA Wei, LI Maohui, AN Changcong, HUANG Yucai. Synthesis and characterization of silico-manganese slag zeolite [J]. Inorganic Chemicals Industry, 2023, 55(12): 128-132. |
| [15] | TENG Jiayang, FENG Qingge, ZHANG Xuan, QIN Fanghong, FENG Jinghang, HU Jiawen, CHEN Chaohong. Study on preparation of pseudo-boehmite from aluminum dross resource treatment [J]. Inorganic Chemicals Industry, 2023, 55(11): 130-138. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||