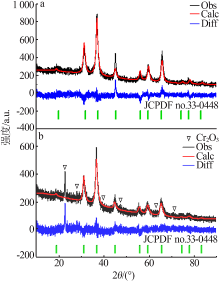
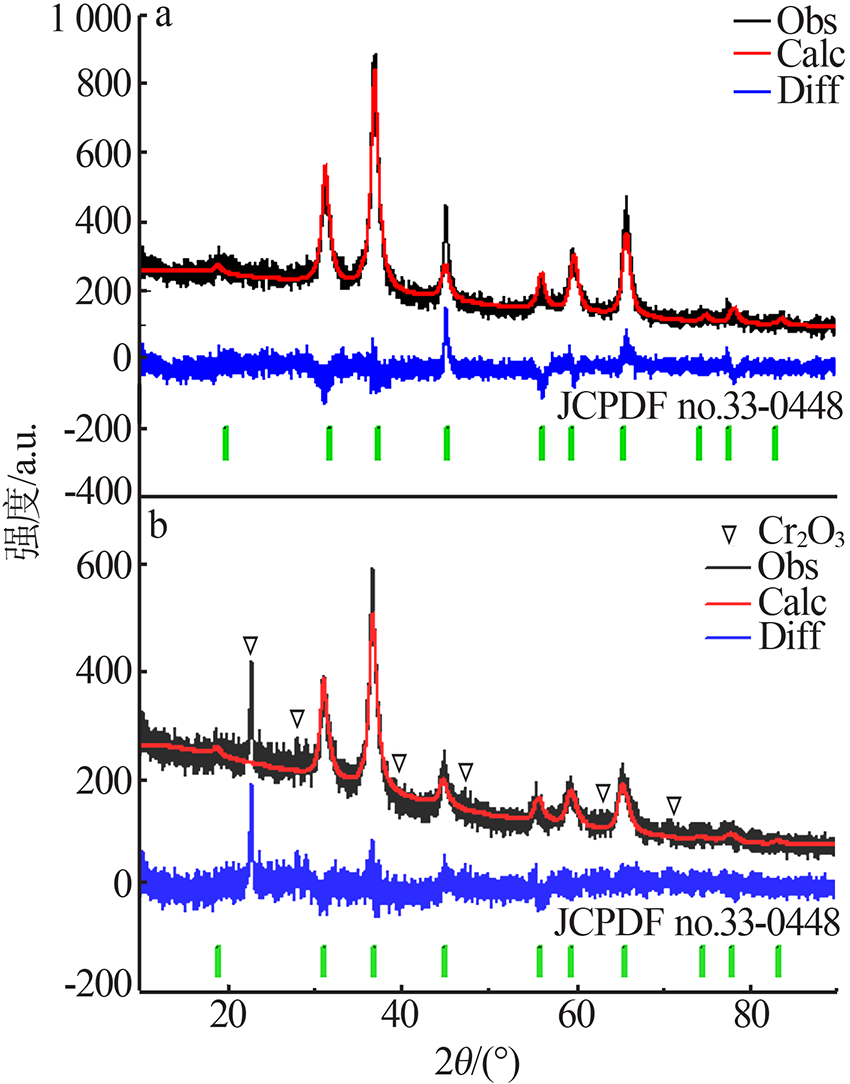
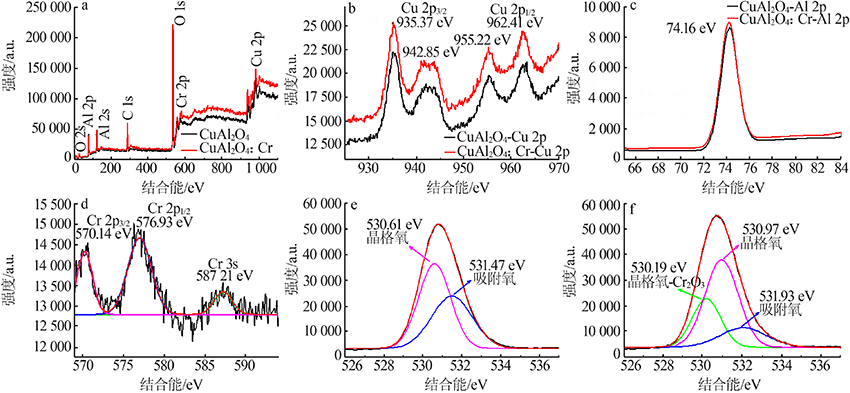
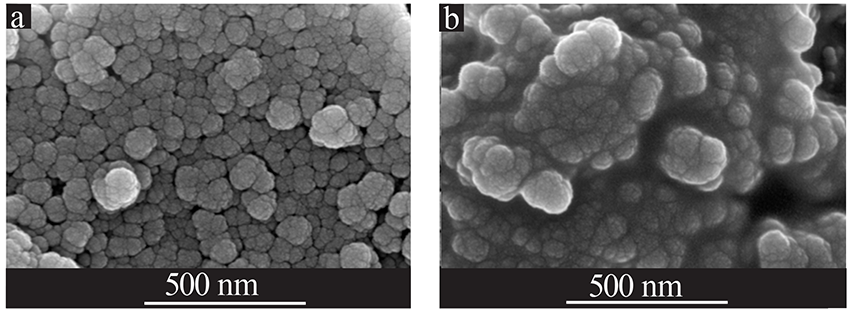
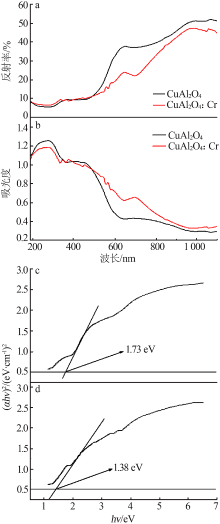
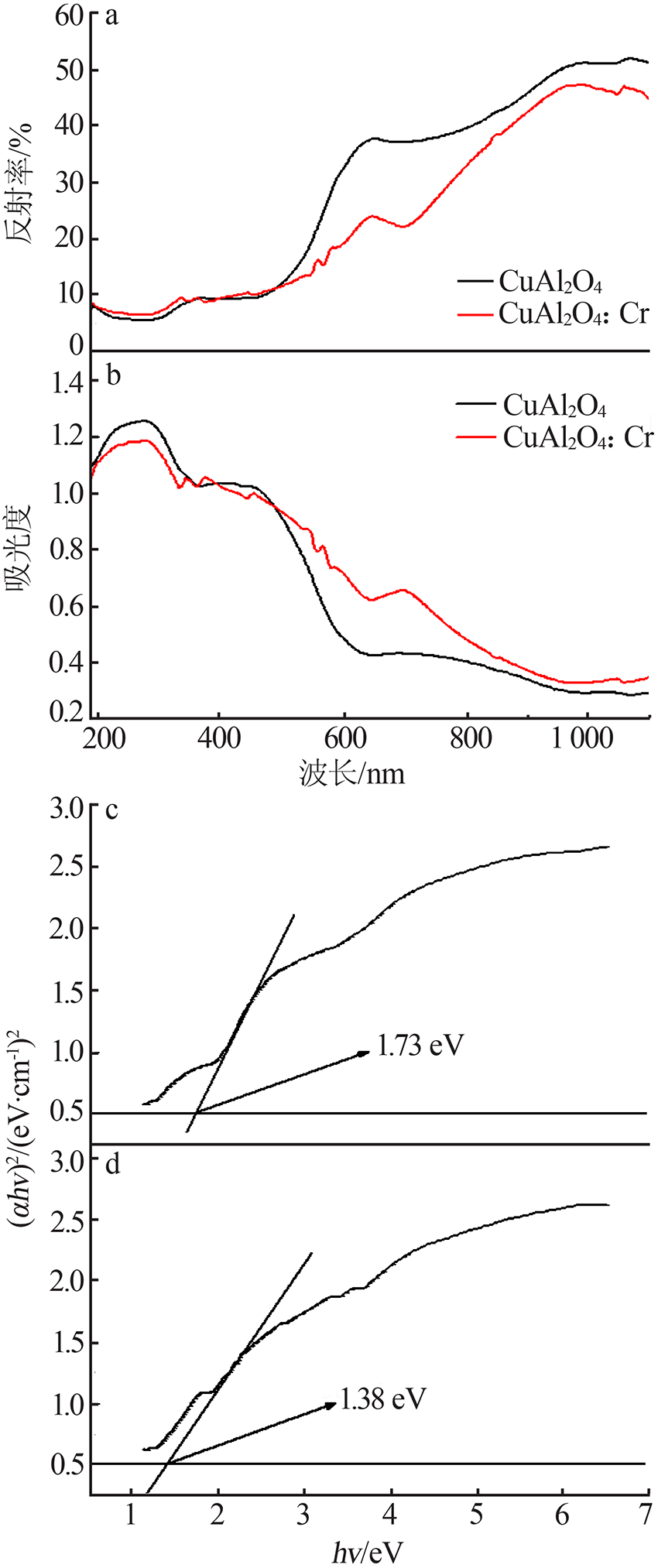
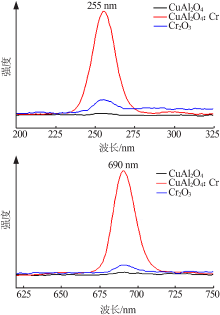
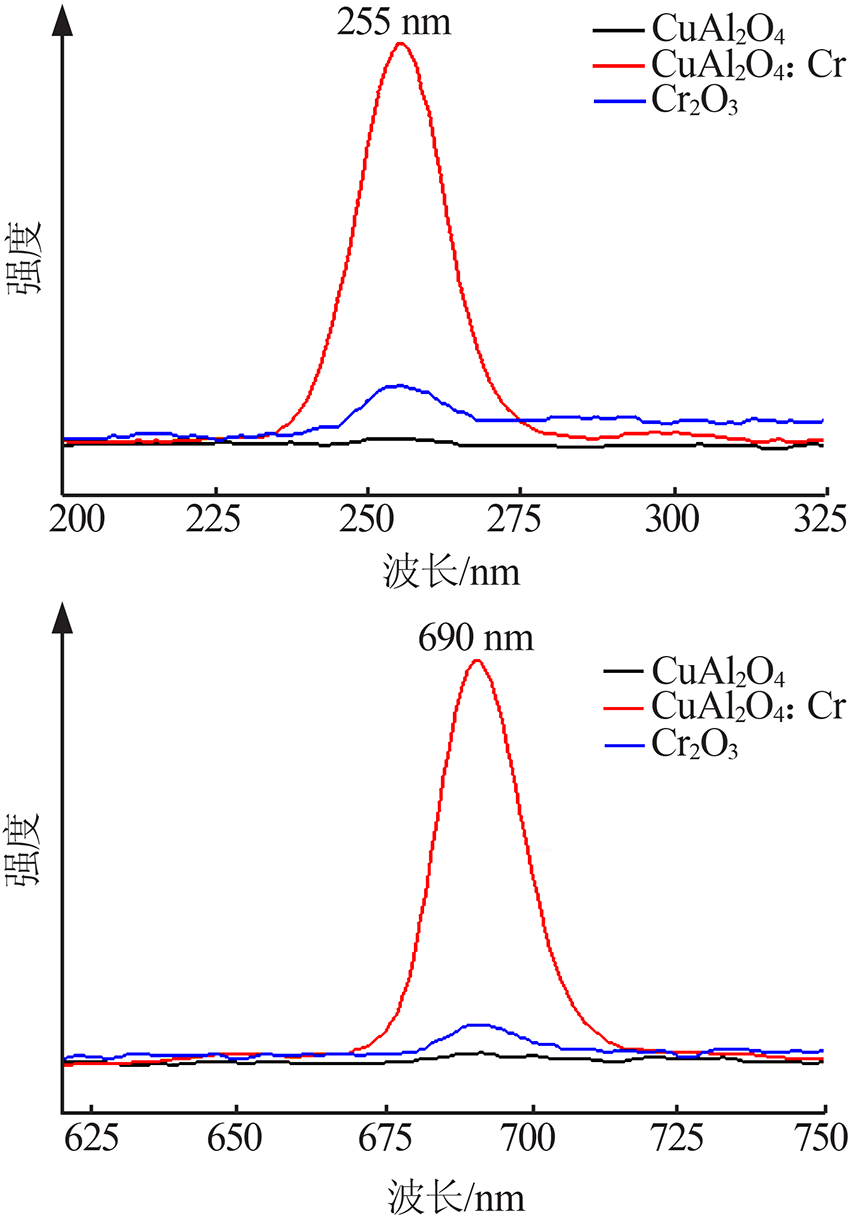
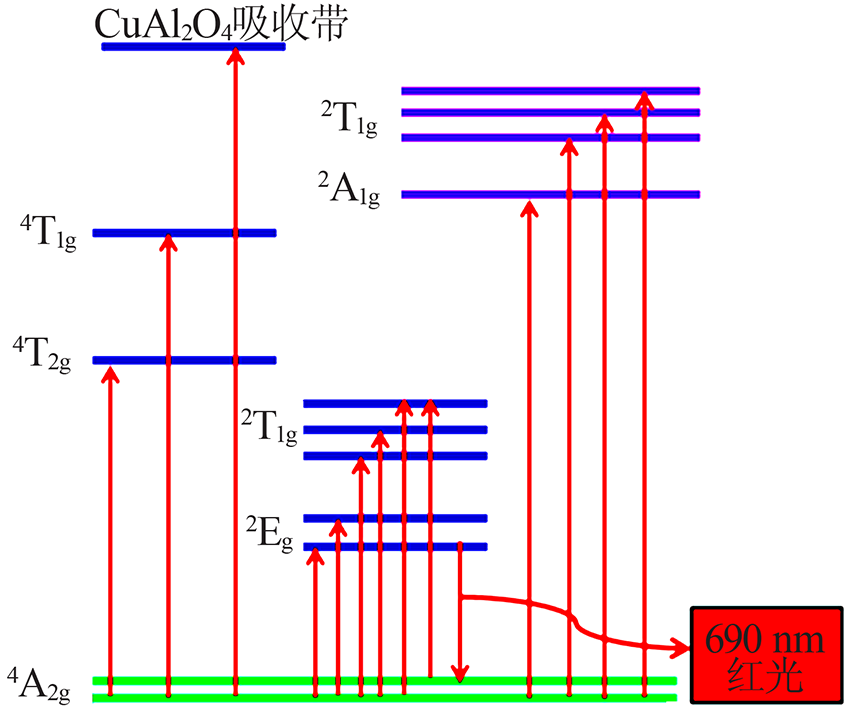
| [1] |
赵文卿, 关荣锋, 王杏. 稀土荧光粉的制备技术研究与展望[J]. 无机盐工业, 2008, 40(10):8-11.
|
| [2] |
杨晓红. MgAl2O4:Ce荧光粉辐照合成及发光机理研究[J]. 无机盐工业, 2019, 51(9):30-35.
|
| [3] |
RYU H, BARTWAL K S. Investigations on luminescence character-istics of Eu and Cr codoped BaAl2O4[J]. Materials Chemistry and Physics, 2008, 111:186-189.
doi: 10.1016/j.matchemphys.2008.04.007
|
| [4] |
葛东来, 范迎菊, 尹龙, 等. 连续在线原位ATR-FTIR技术测定介孔CuAl2O4对黄药的吸附[J]. 物理化学学报, 2013(2):149-154.
|
| [5] |
LV W, LIU B, QIU Q, et al. Synjournal,characterization and photoca-talytic properties of spinel CuAl2O4 nanoparticles by a sonochemical method[J]. Journal of Alloys and Compounds, 2009, 479:480-483.
doi: 10.1016/j.jallcom.2008.12.111
|
| [6] |
TANGCHAROEN T, THIENPRASERT J T, KONGMARK C. Effect of calcination temperature on structural and optical properties of MAl2O4 (M=Ni,Cu,Zn)aluminate spinel nanoparticles[J]. Advan-ced Ceramics, 2019, 3:352-366.
|
| [7] |
HA L H, LANH P T, LONG N N, et al. Some physical properties of ZnAl2O4:Cr3+(Co2+) powders prepared by hydrothermal method[J]. Journal of Physics:Conference Series, 2009, 187.Doi: 10.1088/1742-6596/187/1/012053.
doi: 10.1088/1742-6596/187/1/012053
|
| [8] |
MENON S G, HEBBAR D N, KULKARNI S D, et al. Facile synthe-sis and luminescence studies of nanocrystalline red emitting Cr:ZnAl2O4 phosphor[J]. Materials Research Bulletin, 2017, 86:63-71.
doi: 10.1016/j.materresbull.2016.10.009
|
| [9] |
朱金剑, 张景成, 南军, 等. EDTA/CA对加氢处理催化剂性能的影响[J]. 无机盐工业, 2019, 51(7):85-88.
|
| [10] |
TENG Y, ZHOU J, KHISRO S N, et al. Persistent luminescence of SrAl2O4:Eu2+,Dy3+,Cr3+ phosphors in the tissue transparency win-dow[J]. Materials Chemistry and Physics, 2014, 147:772-776.
doi: 10.1016/j.matchemphys.2014.06.018
|
| [11] |
PONMUDI S, SIVAKUMAR R, SANJEEVIRAJA C, et al. Facile fabrication of spinel structured n-type CuAl2O4 thin film with nano-grass like morphology by sputtering technique[J]. Applied Surfa-ce Science, 2019, 483:601-615.
|
| [12] |
KOCHUR A G, KOZAKOV A T, GOOGLEV K A, et al. Valence state of transition metal ions in Co1-xFexCr2O4(x=0.1,0.2,0.5) ce-ramics from X-ray photoelectron and Mössbauer spectroscopy data[J]. Journal of Alloys and Compounds, 2015, 636:241-248.
doi: 10.1016/j.jallcom.2015.02.150
|
| [13] |
周元, 刘素芹, 梁英. 稳定的高可见光活性Ag3PO4/WO3复合光催化剂[J]. 应用化学, 2013, 30(6):673-676.
|
| [14] |
LOU F H, BALLEXTYNE D W G. Visible and ultra-violet emission and absorption spectra of MgAl2O4:Cr[J]. Journal of Physics C:So-lid State Physics, 1968, 2(1):608-613.
|