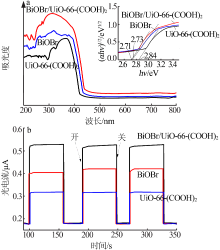
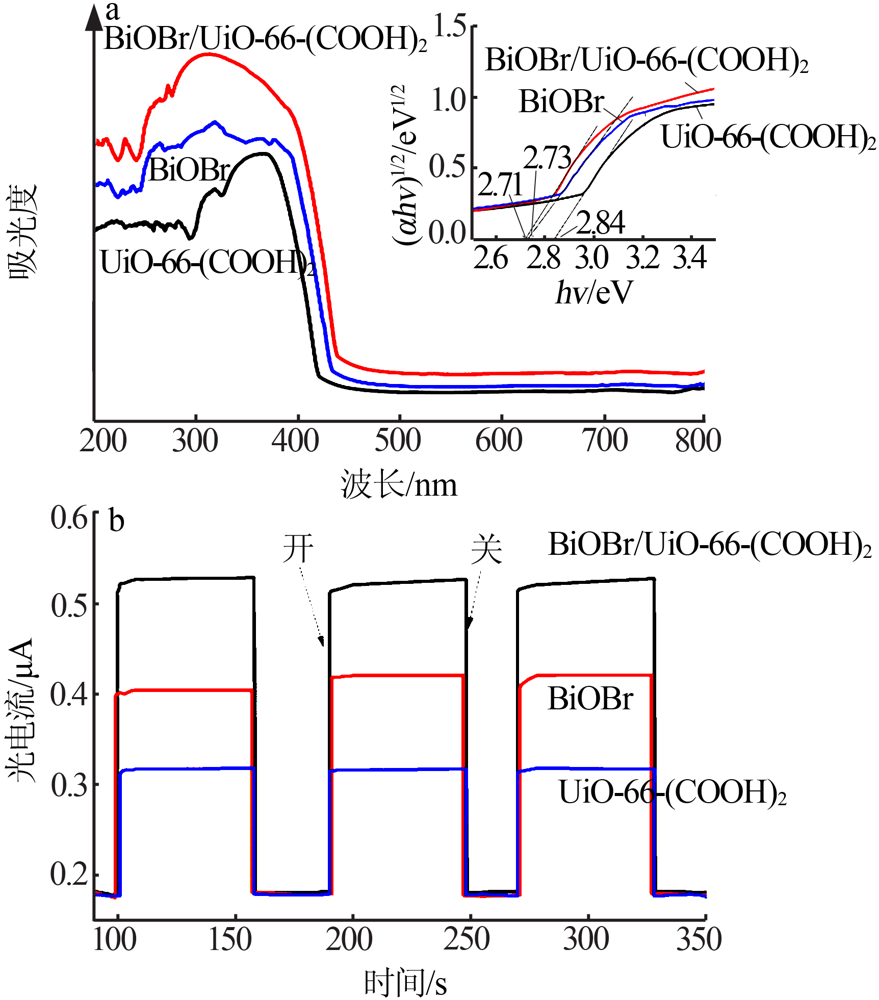
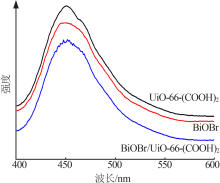
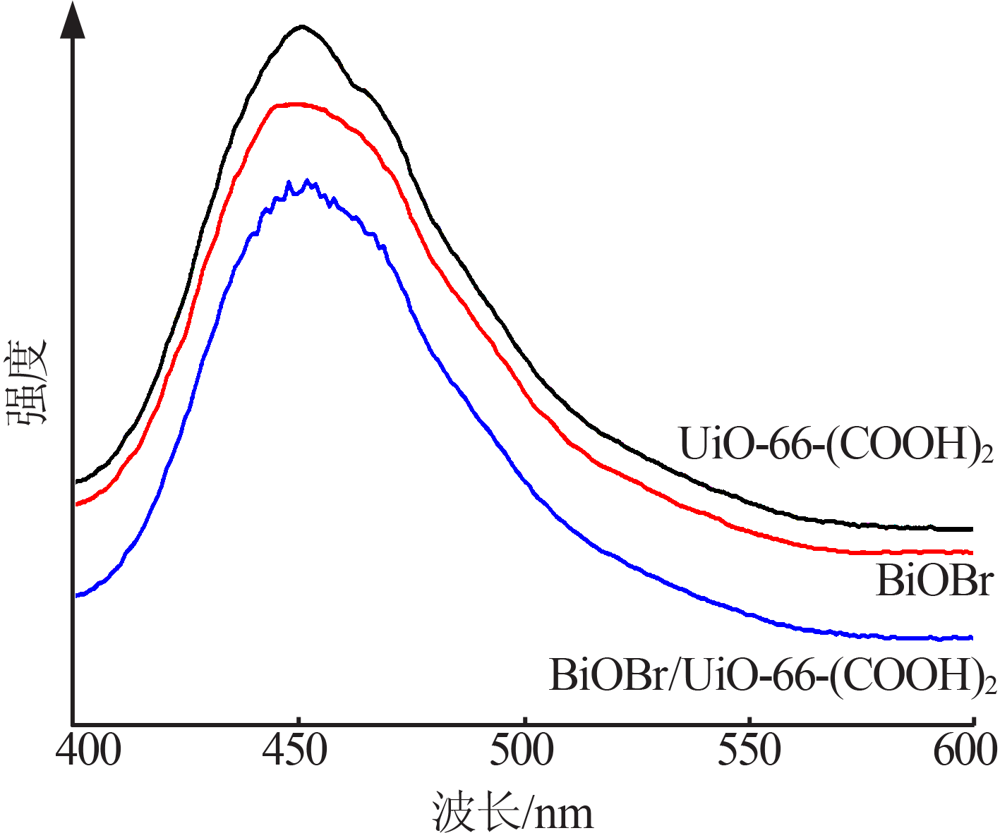
| [1] |
ZHAO Wanyue, DING Tong, WANG Yating, et al. Decorating Ag/ AgCl on UiO-66-NH2:Synergy between Ag plasmons and hetero- structure for the realization of efficient visible light photocataly- sis[J]. Chinese Journal of Catalysis, 2019, 40(8):1187-1197.
doi: 10.1016/S1872-2067(19)63377-2
|
| [2] |
LIMVORAPITUX Rungmai, CHEN Haoyuan, MENDONCA Ma- tthew, et al. Elucidating the mechanism of the UiO-66-catalyzed sulfide oxidation:activity and selectivity enhancements through ch- anges in the node coordination environment and solvent[J]. Cataly- sis Science & Technology, 2018, 9(2):327-335.
|
| [3] |
LUO Tian, ZHANG Jianling, LI Wei, et al. Metal-organic framework stabilized CO2/water interfacial route for photocatalytic CO2 conver- sion[J]. ACS Applied Materials & Interfaces, 2017, 9:41594-41598.
|
| [4] |
陈小浪. 新型光催化纳米材料的制备及其氧化NO和产氢性能的研究[D]. 上海:上海师范大学, 2018.
|
| [5] |
冯飞, 李书文, 汪铁林, 等. 片状铋/钒酸铋复合催化剂的制备及其光催化性能[J]. 无机盐工业, 2021, 53(1):107-112.
|
| [6] |
冯胜雷, 刘方华, 付翔, 等. TiO2/g-C3N4光催化材料的制备及其可见光降解性能研究[J]. 化工新型材料, 2020, 48(11):99-102,107.
|
| [7] |
CAO Jing, XU Benyan, LUO Bangde, et al. Novel BiOI/BiOBr het- erojunction photocatalysts with enhanced visible light photocatalytic properties[J]. Catalysis Communications, 2011, 13(1):63-68.
doi: 10.1016/j.catcom.2011.06.019
|
| [8] |
GUO Junqiu, LIAO Xin, LEE Ming-Hsien, et al. Experimental and DFT insights of the Zn-doping effects on the visible-light photoca- talytic water splitting and dye decomposition over Zn-doped BiOBr photocatalysts[J]. Applied Catalysis B:Environmental, 2018, 243:502-512.
doi: 10.1016/j.apcatb.2018.09.089
|
| [9] |
KONG Liang, JIANG Zheng, LAI Henry H, et al. Unusual reactivity of visible-light-responsive AgBr-BiOBr heterojunction photocataly- sts[J]. Journal of Catalysis, 2012, 293:116-125.
doi: 10.1016/j.jcat.2012.06.011
|
| [10] |
ZHANG Xinxin, LI Ruiping, JIA Manke, et al. Degradation of cipro- floxacin in aqueous bismuth oxybromide (BiOBr) suspensions un- der visible light irradiation:A direct hole oxidation pathway[J]. Chemical Engineering Journal, 2015, 274:290-297.
doi: 10.1016/j.cej.2015.03.077
|
| [11] |
ZHANG Xi, ZHANG Lizhi, XIE Tengfeng, et al. Low-temperature synjournal and high visible-light-induced photocatalytic activity of BiOI/TiO2 heterostructures[J]. The Journal of Physical Chemistry C, 2009, 113(17):7371-7378.
doi: 10.1021/jp900812d
|
| [12] |
于吉行, 俞俊, 薛晓雅, 等. 金属有机骨架UiO-66在催化领域的应用[J]. 化工进展, 2019, 38(增刊1):144-151.
|
| [13] |
WANG Rong, GU Lina, ZHOU Jianjian, et al. Quasi-polymeric me- tal-organic framework UiO-66/g-C3N4 heterojunctions for enhan- ced photocatalytic hydrogen evolution under visible light irradia- tion[J]. Advanced Materials Interfaces, 2015, 2(10).Doi: 10.1002/admi.201500037.
doi: 10.1002/admi.201500037
|
| [14] |
YE Liqun, LIU Jinyan, JIANG Zhuo, et al. Facets coupling of BiOBr- g-C3N4 composite photocatalyst for enhanced visible-light-driven photocatalytic activity[J]. Applied Catalysis B:Environmental, 2013, 142-143(Complete):1-7.
doi: 10.1016/j.apcatb.2013.04.058
|
| [15] |
CHAVAN Sachin M, SHEARER Greig C, SVELLE Stian, et al. Sy njournal and characterization of amine-functionalized mixed-ligand metal-organic frameworks of UiO-66 topology[J]. Inorganic Chemi- stry, 2014, 53(18):9509-9523.
|
| [16] |
SINGH Pardeep, SONU, RAIZADA Pankaj, et al. Enhanced photo- catalytic activity and stability of AgBr/BiOBr/graphene heterojunc- tion for phenol degradation under visible light[J]. Journal of Saudi Chemical Society, 2019, 23(5):586-599.
doi: 10.1016/j.jscs.2018.10.005
|
| [17] |
RAGON Florence, CAMPO Betiana, YANG Qingyuan, et al. Acic- functionalized UiO-66(Zr) MOFs and their evolution after intra- framework cross-linking:Structural features and sorption properti- es[J]. Journal of Materials Chemistry A, 2015, 3(7):3294-3309.
doi: 10.1039/C4TA03992K
|
| [18] |
任勇, 潘越, 刘德蓉, 等. Rh/UiO-66-NH2催化1,4-丁炔二醇加氢性能研究[J]. 应用化工, 2019, 48(1):136-139,144.
|
| [19] |
朱帅汝. BiOBr/MOF复合材料的制备与光催化性能的研究[D]. 宁波:宁波大学, 2018.
|
| [20] |
杨黄根, 陈渊, 王治伟, 等. 多孔柿饼状BiOBr光催化剂的简易溶剂热法合成[J]. 无机化学学报, 2020, 36(2):333-344.
|
| [21] |
ZHU Guiliang, SHENG Feng, SHAO Cong, et al. One-pot synjournal of C-dots modified TiO2 nanosheets/UiO-66-NH2 with improved photocatalytic activity under visible light[J]. Ceramics Internatio- nal, 2020, 46(2):2530-2537.
|
| [22] |
YAN Yuxiang, YANG Hua, YI Zao, et al. Direct Z-scheme CaTiO3@BiOBr composite photocatalysts with enhanced photodegradation of dyes[J]. Environmental Science and Pollution Research, 2019, 26(28):29020-29031.
doi: 10.1007/s11356-019-06085-y
|
 ),CHEN Xin2,ZHANG Jing2,DENG Niyan2,ZHANG Quan2,LIU Shengpeng1,2,SUN Guofeng1,2(
),CHEN Xin2,ZHANG Jing2,DENG Niyan2,ZHANG Quan2,LIU Shengpeng1,2,SUN Guofeng1,2( )
)