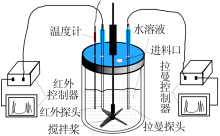
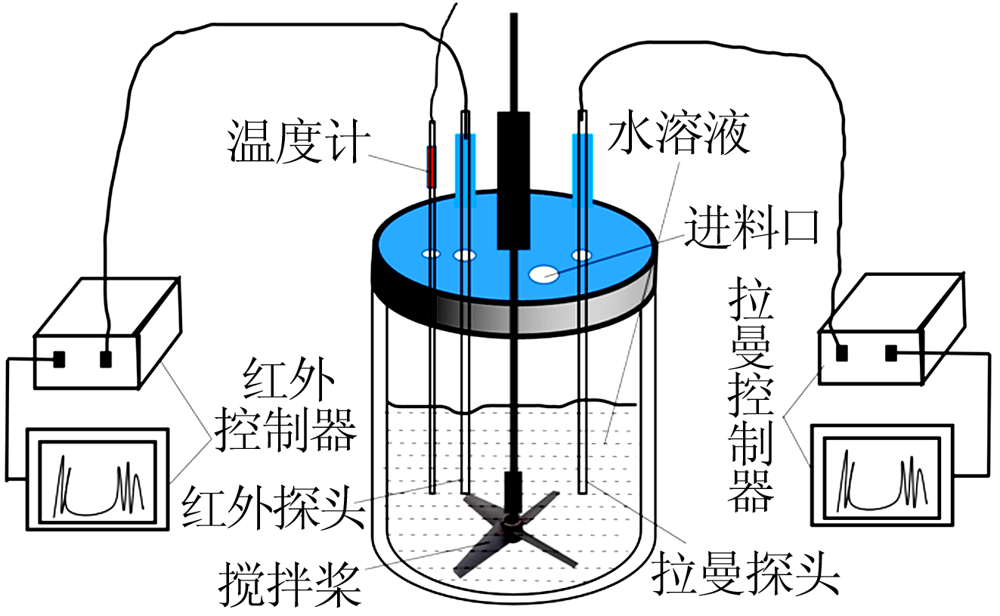
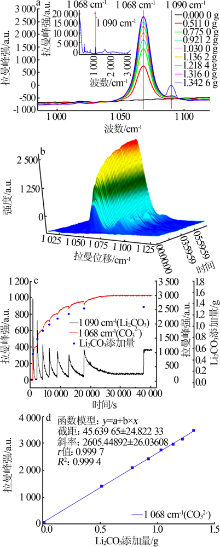
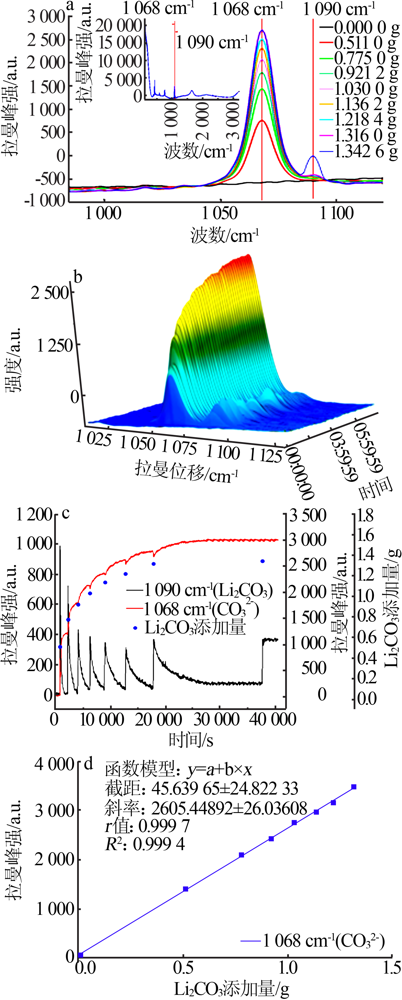
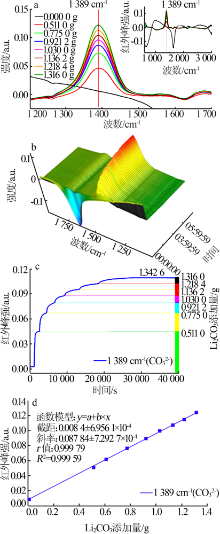
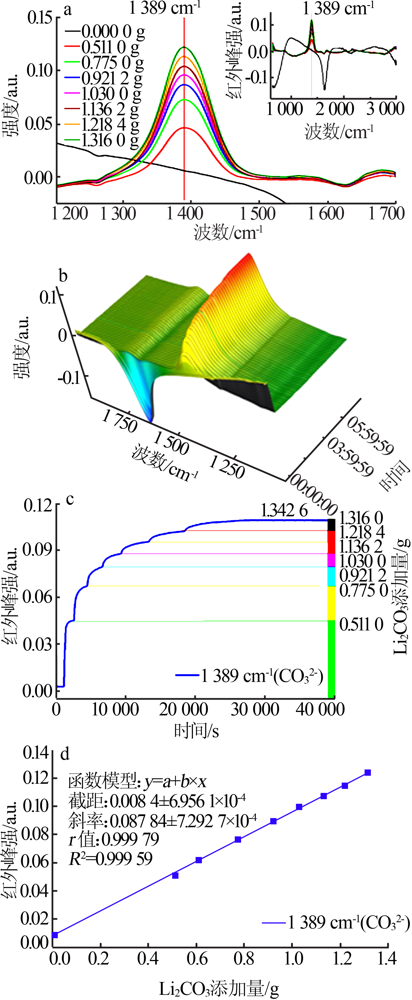
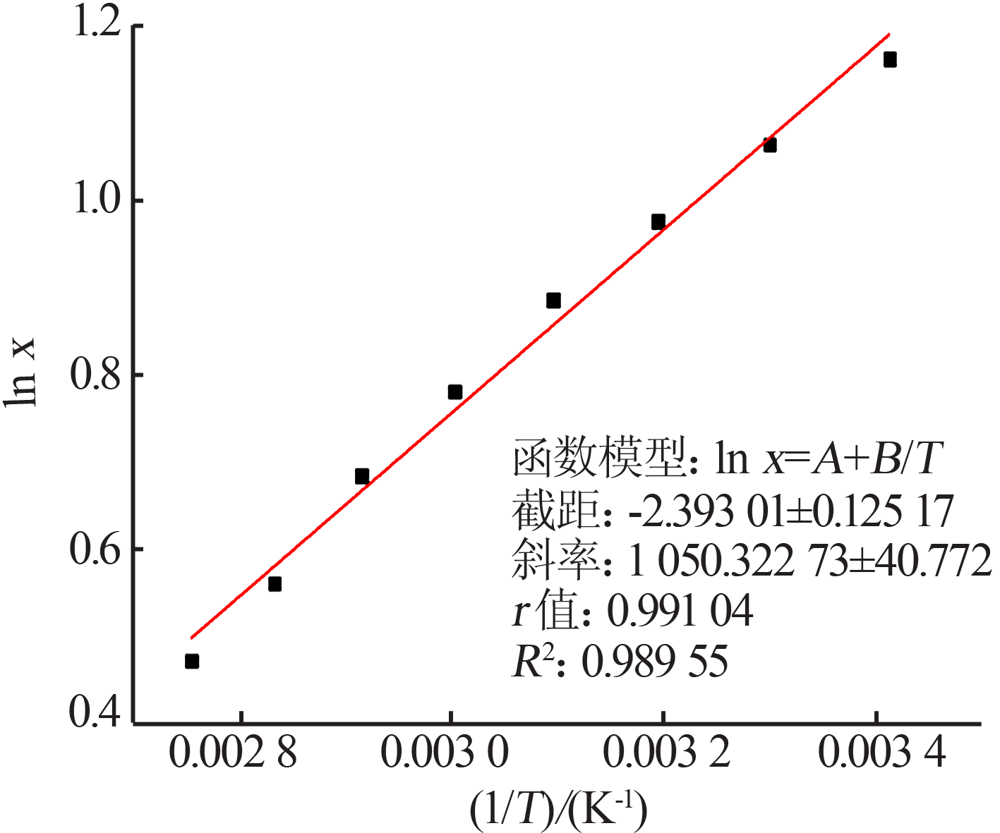
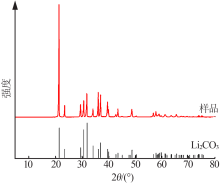
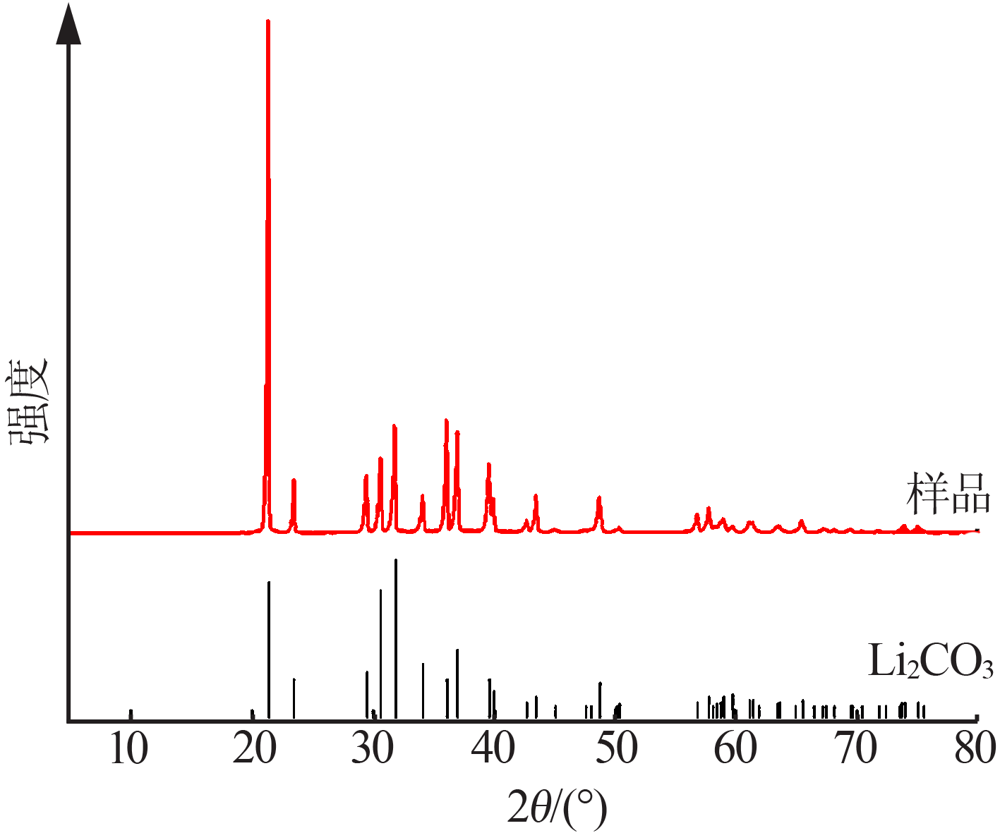
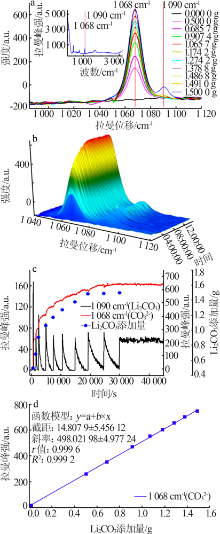
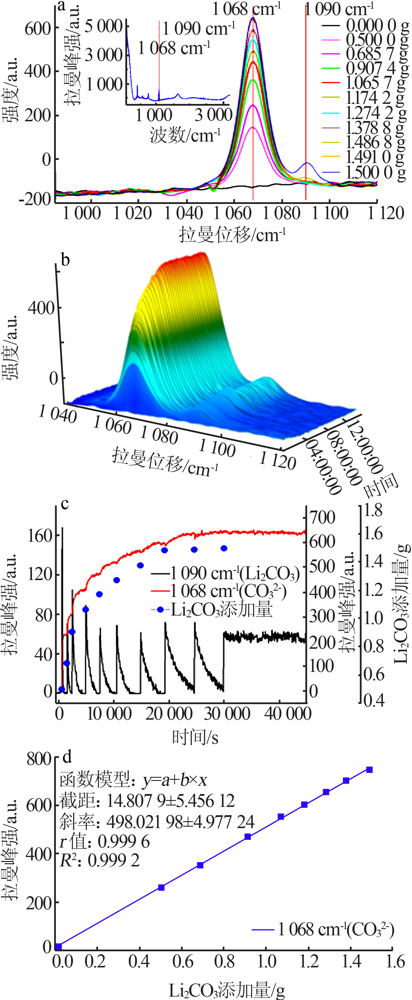
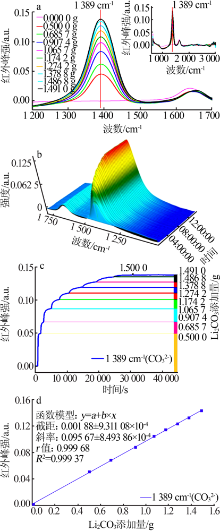
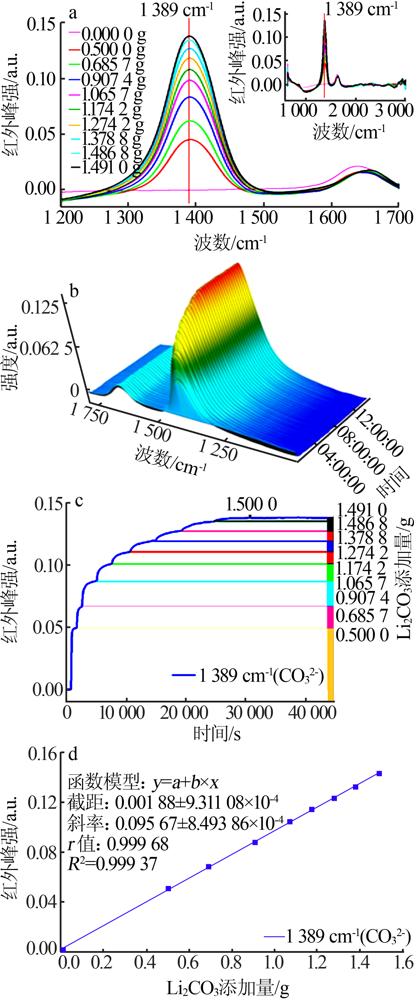
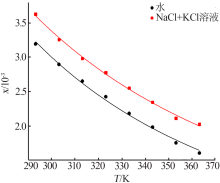
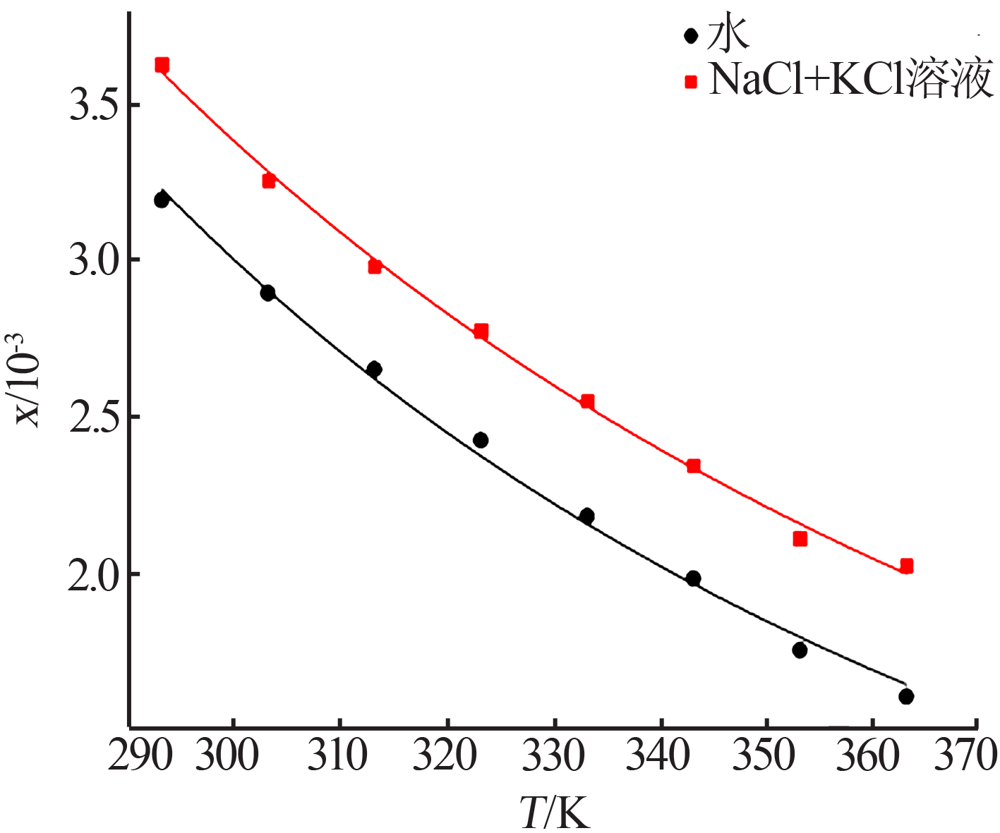
| [1] |
Pan Xijuan, Dou Zhihe, Zhang Tingan, et al. Basic study on direct preparation of lithium carbonate powders by membrane electroly-sis[J]. Hydrometallurgy, 2020, 191.Doi: 10.1016/j.hydromet.2019.105193.
|
| [2] |
Wang Yan, Du Shichao, Wang Xuemei, et al. Spherulitic growth and morphology control of lithium carbonate:the stepwise evolution of core-shell structures[J]. Powder Technology, 2019, 355:617-628.
doi: 10.1016/j.powtec.2019.07.061
|
| [3] |
Kadam S S, Kulkarni S A, Ribera R C, et al. A new view on the me-tastable zone width during cooling crystallization[J]. Chemical En-gineering Science, 2012, 72:10-19.
|
| [4] |
孙玉柱. 碳酸锂结晶过程研究[D]. 上海:华东理工大学, 2010.
|
| [5] |
Guan Qian, Liu Yong, Ling Bo, et al. Effect of magnetic field on so-dium arsenate metastable zone width and crystal nucleation kinetics for crystallization[J]. International Journal of Chemical Kinetics, 2020, 52(7).Doi: 10.1002/kin.21362.
|
| [6] |
宋昌斌, 李润超. 碳酸锂在水中的溶解度和超溶解度的测定及热力学分析[J]. 化工进展, 2016, 35(8):2350-2354.
|
| [7] |
戈海文, 王怀有, 王敏. 碳酸锂在碳酸钠溶液中的溶解度与热力学[J]. 化工学报, 2019, 70(11):4123-4130.
|
| [8] |
Wang H Y, Du B Q, Wang M. Study of the solubility,supersolubility and metastable zone width of Li2CO3 in the LiCl-NaCl-KCl-Na2SO4 system from 293.15 to 353.15 K[J]. Journal of Chemical & Engin-eering Data, 2018, 63(5):1429-1434.
|
| [9] |
Cheng W T, Li Z B, Cheng F Q. Solubility of Li2CO3 in Na-K-Li-Cl brines from 20 to 90 ℃[J]. J.Chem.Thermodynamics, 2013, 67:74-82.
|
| [10] |
张莉媛, 王刚, 白树宽, 等. 表面活性剂对氯化钾结晶介稳区和诱导期测定的影响[J]. 盐科学与化工, 2020, 49(3):35-39.
|
| [11] |
Nishinaga T. Handbook of crystal growth[M]. Amsterdam:Elsevier, 2015.
|
| [12] |
李泽慧. 谷氨酸钠悬浊体系的拉曼光谱定量分析[J]. 当代化工研究, 2020(5):48-51.
|
| [13] |
李敏章. 偶氮二异丁酸二甲酯的结晶过程研究[D]. 广州:华南理工大学, 2018.
|
| [14] |
刘光启, 马连湘, 项曙光. 化学化工特性数据手册.无机卷[M]. 北京: 化学工业出版社, 2013: 329.
|
| [15] |
陈俊波, 符秀娟. 近红外光谱技术在药物分析中的应用[J]. 医学食疗与健康, 2020, 18(13):160-161,163.
|
| [16] |
王玉娟. 近红外光谱分析技术在化工分析领域的应用探讨[J]. 化工管理, 2019(21):113-114.
|
| [17] |
李春哲. 近红外光谱分析技术在化工分析领域的应用[J]. 花炮科技与市场, 2018(4):170-171.
|
| [18] |
余璐. 近红外光谱分析技术在化工领域的研究应用[J]. 化工管理, 2017(24):136.
|
| [19] |
陶箴奇. 以盐湖碳酸锂为原料制备电池级碳酸锂的研究[D]. 西宁:青海大学, 2016.
|
| [20] |
朱佳兵, 钟辉, 刘善东, 等. 硫酸钙在高温盐溶液中的溶解度[J]. 化工技术与开发, 2015, 44(12):13-14.
|
 ),Deng Xiaochuan1,2(
),Deng Xiaochuan1,2( ),Shi Yifei1,2,Dong Chaochao1,2,3,Fan Faying1,2,Zhu Chaoliang1,2,Fan Jie1,2
),Shi Yifei1,2,Dong Chaochao1,2,3,Fan Faying1,2,Zhu Chaoliang1,2,Fan Jie1,2