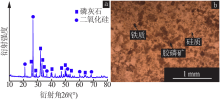
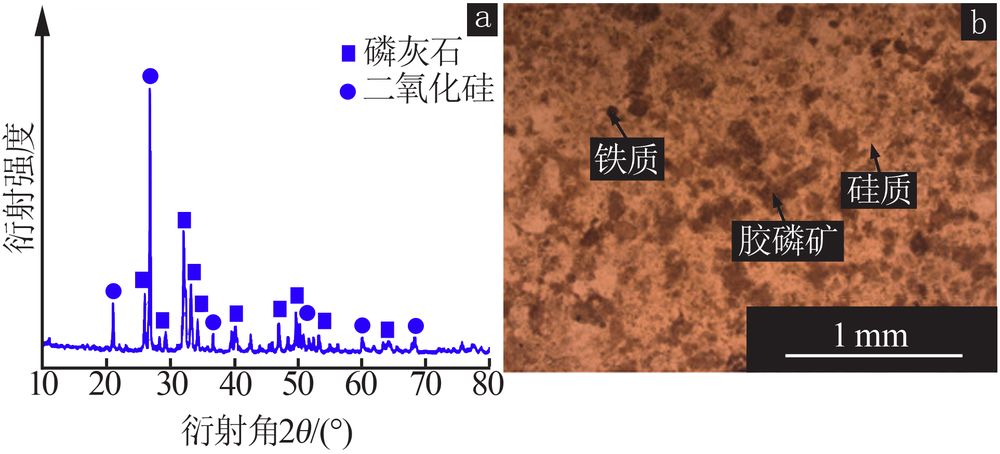
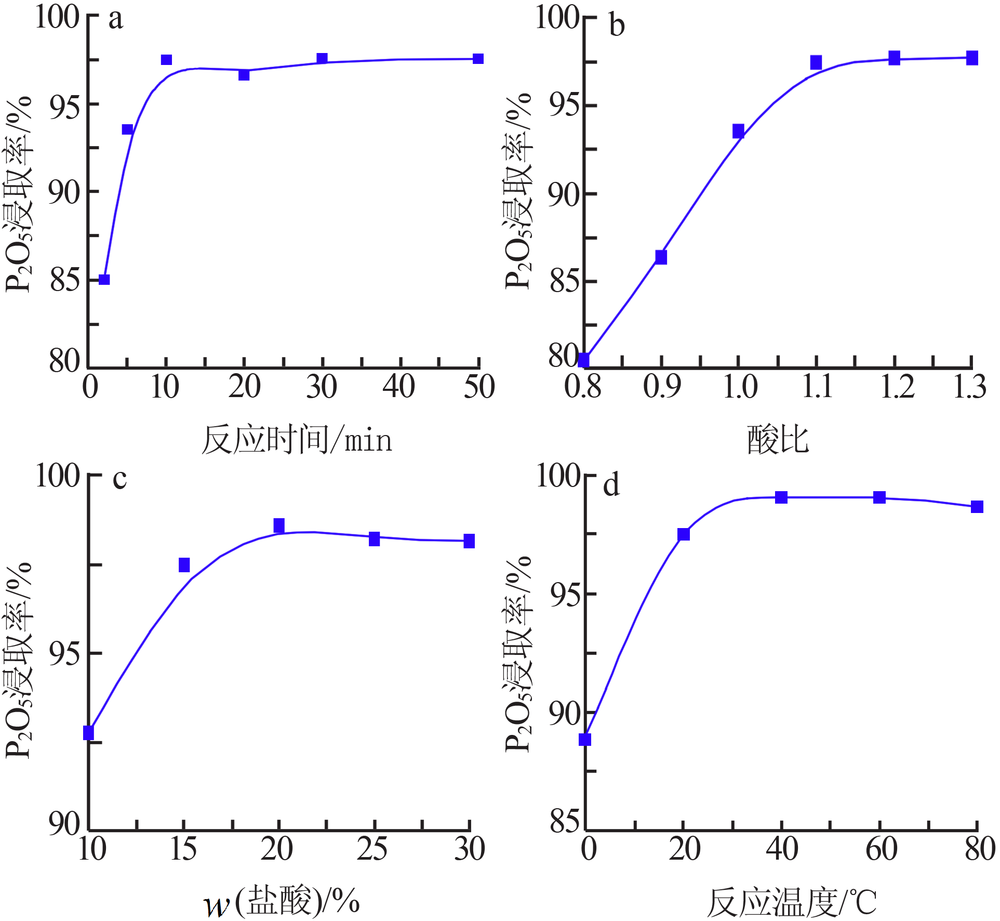
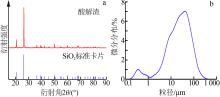
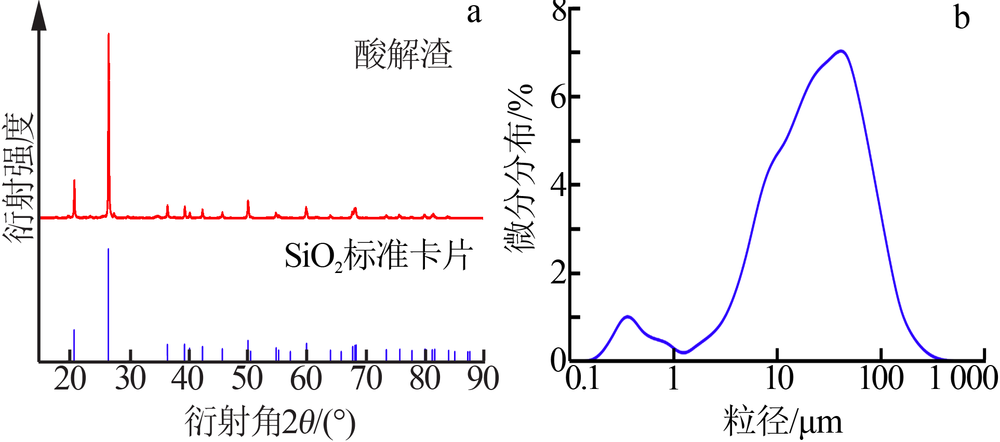
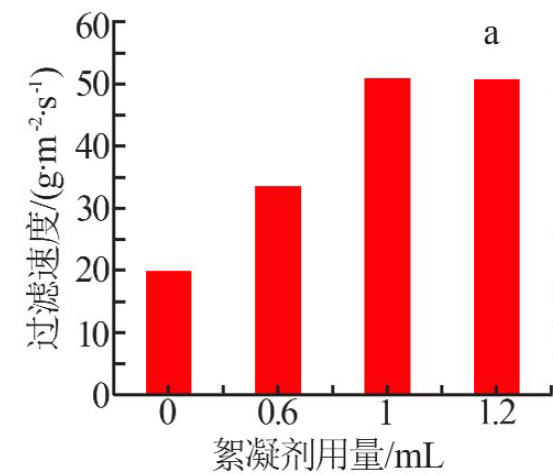
| [1] |
YU Zhou, HE Zhaoyi, TANG Liang, HE Sheng, XIAO Haixin, XIAO Yixun.
Study on preparation and microscopic properties of typical sulfate solid waste composite cementitious materials
[J]. Inorganic Chemicals Industry, 2024, 56(4): 90-97.
|
| [2] |
WANG Jianrui, ZHANG Song, ZHANG Jie.
Study on new process for beneficiation phosphate of low-grade phosphate rock leaching via lactic acid
[J]. Inorganic Chemicals Industry, 2024, 56(3): 56-63.
|
| [3] |
WANG Ruting, ZHAO Xiaorong, HUANG Xuquan, WANG Haojie, XUE Fei, CAI Jiawei.
Research on preparation and early performance of mixed phase phosphogypsum-based cementing materials
[J]. Inorganic Chemicals Industry, 2024, 56(3): 98-104.
|
| [4] |
HUANG Tao, HUANG Zili, XIAO Shuo, ZHENG Jiemiao, LIU Xiaofeng, WU Jilong.
Experimental study on preparation of polyferric chloride from iron tailings acid leaching solution
[J]. Inorganic Chemicals Industry, 2024, 56(2): 121-126.
|
| [5] |
XIA Guiying, YANG Liuchun, YUAN Zhiye.
Study on direct leaching of rare earth elements from phosphogypsum with sulfuric acid
[J]. Inorganic Chemicals Industry, 2024, 56(1): 107-113.
|
| [6] |
XIANG Mengqi, MENG Hua, WANG Ye, MENG Xianzhang, BAI Yuhang, WANG Yujunyao, ZHANG Yidan.
Study on kinetic of iron leaching from titanium gypsum and its cyclic acid leaching process
[J]. Inorganic Chemicals Industry, 2024, 56(1): 114-120.
|
| [7] |
ZHANG Conghua, YAN Wenbin, XIAO Jiajun, ZHAO Ke, PENG Shangquan, WEI Yuhong.
Reductive leaching technology of manganese anode slag using tartaric acid as reducing agent optimized by RSM
[J]. Inorganic Chemicals Industry, 2023, 55(9): 106-113.
|
| [8] |
LUO Wenbo, LI Heng, LÜ Jun, YANG Linguang, ZHAO Xingfan, LONG Xiao.
Study on recovery of silicon and aluminum from industrial silicon slag
[J]. Inorganic Chemicals Industry, 2023, 55(9): 94-99.
|
| [9] |
DONG Xinyu, WANG Haifeng, HE Yue, YANG Pan, WANG Song, YANG Chunyuan, WANG Qin, HUANG Bifang, WANG Jiawei.
Exleaching toxicity analysis and harmless treatment of electrolytic manganese residue
[J]. Inorganic Chemicals Industry, 2023, 55(5): 85-90.
|
| [10] |
DONG Xiongwei, HAN Fenglan, HUA Wei, LI Maohui, AN Changcong, HUANG Yucai.
Synthesis and characterization of silico-manganese slag zeolite
[J]. Inorganic Chemicals Industry, 2023, 55(12): 128-132.
|
| [11] |
TENG Jiayang, FENG Qingge, ZHANG Xuan, QIN Fanghong, FENG Jinghang, HU Jiawen, CHEN Chaohong.
Study on preparation of pseudo-boehmite from aluminum dross resource treatment
[J]. Inorganic Chemicals Industry, 2023, 55(11): 130-138.
|
| [12] |
ZHOU Xiaoping, FENG Haoshou, ZHANG Guohui, HOU Shilei, ZHANG Shuaipeng, LIU Lixia.
Development and practice of continuous defluoridation technology for by-products fluorinated hydrochloric acid of lithium hexafluorophosphate
[J]. Inorganic Chemicals Industry, 2023, 55(11): 93-99.
|
| [13] |
LI Huifang, WANG Xiao, BAI Youpeng, ZHANG Shixiang.
Study on extraction of lithium from low grade high clay leaching solution by solvent extraction
[J]. Inorganic Chemicals Industry, 2023, 55(10): 63-69.
|
| [14] |
HU Luoxing,HUANG Qimao,QU Hongyou.
Preparation of Fe3O4 from FeCl2 by-product of new process of titanium dioxide by hydrochloric acid
[J]. Inorganic Chemicals Industry, 2023, 55(1): 118-123.
|
| [15] |
LIU Darui,XU Lijun,LI Shichun,CAO Kun,TU Ya,LI Wenqing,LIU Qingliang.
Research progress of recovery of strategic metal lithium from fly ash
[J]. Inorganic Chemicals Industry, 2023, 55(1): 56-63.
|
 )
)