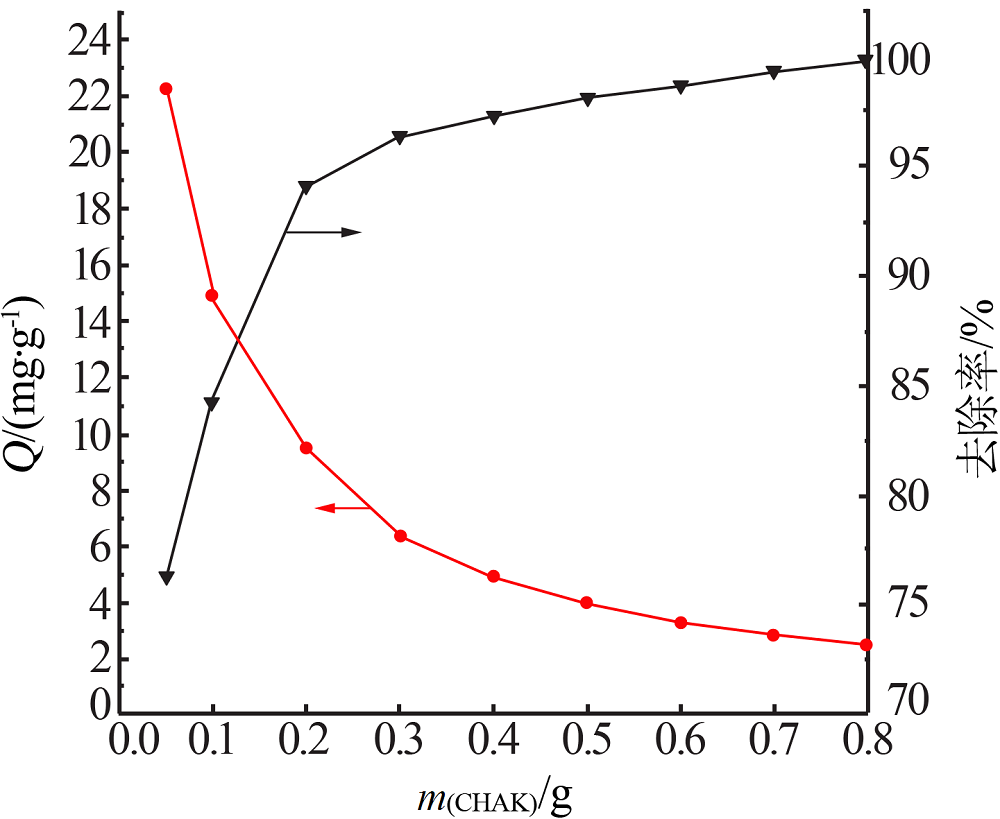
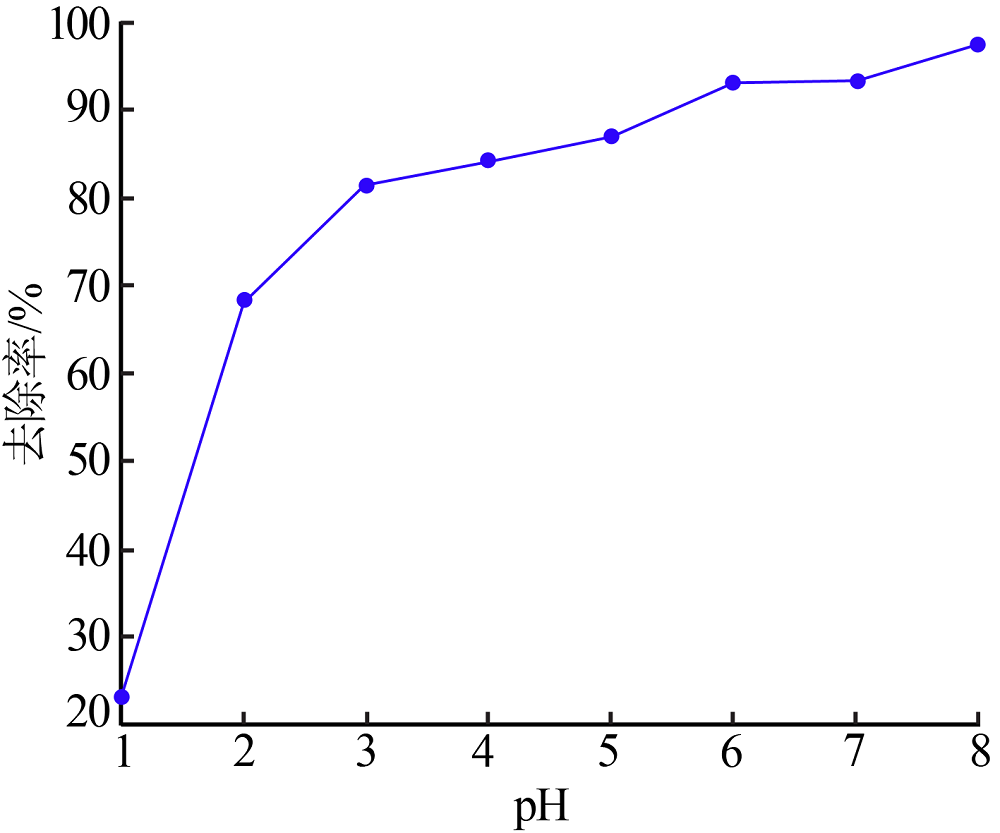
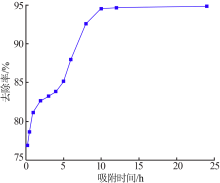
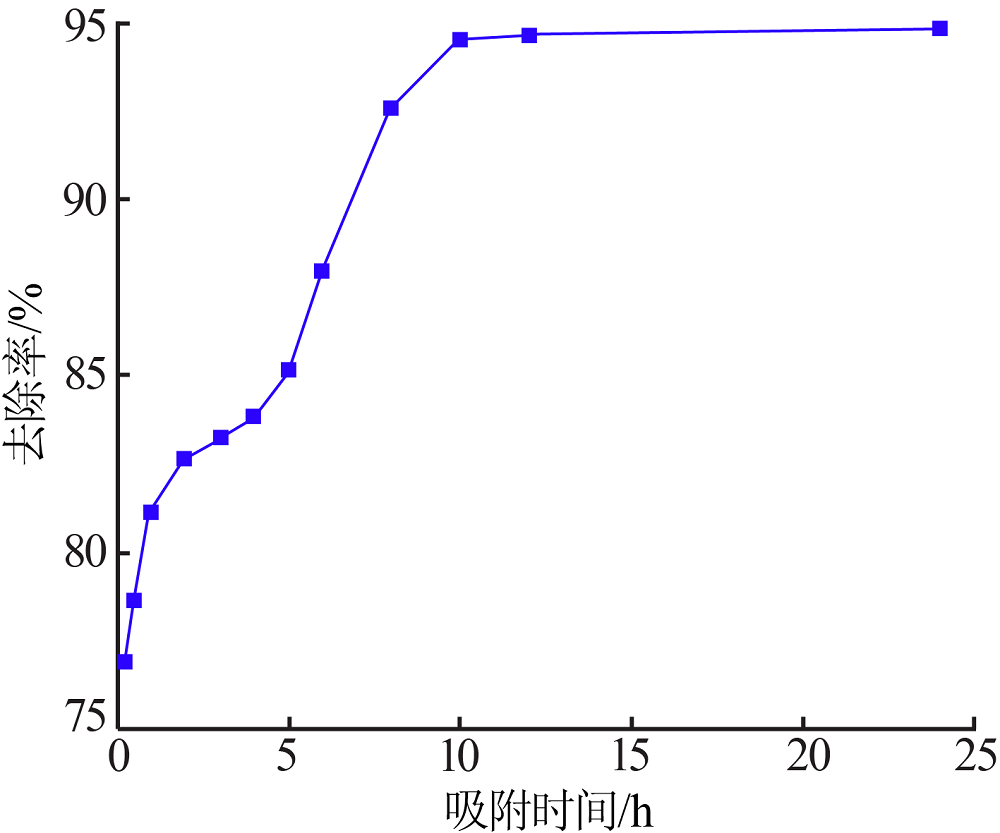
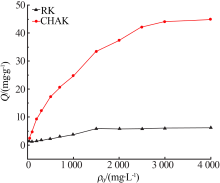
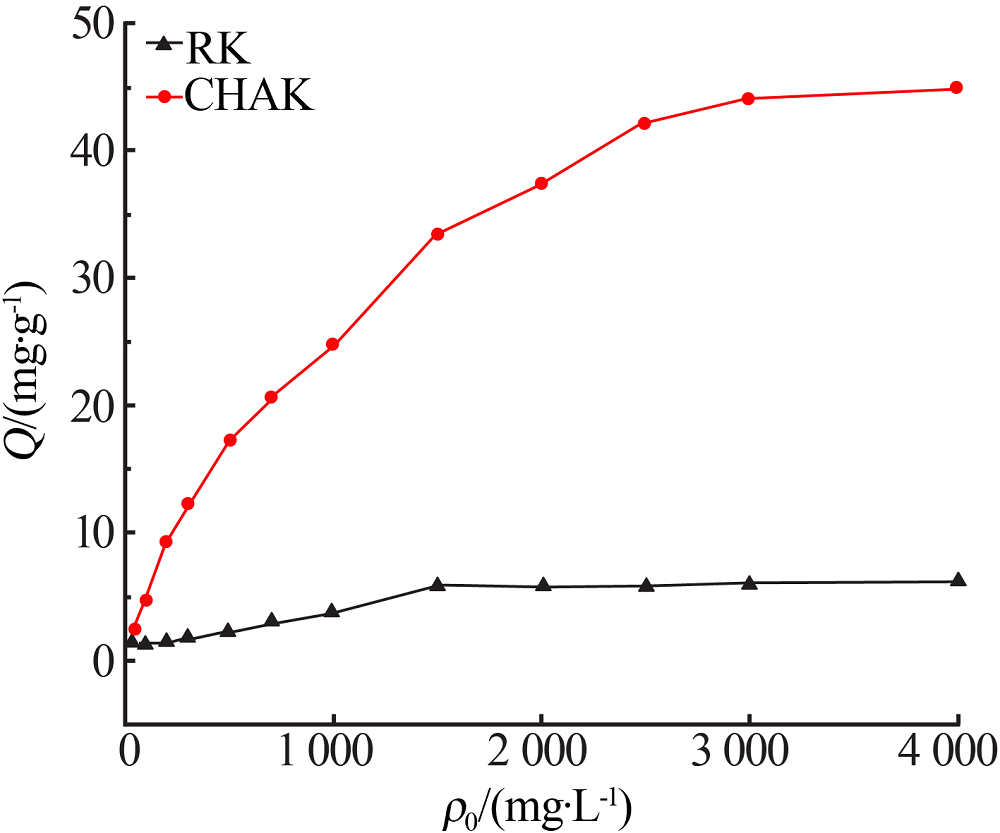
Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (2): 30-34.doi: 10.11962/1006-4990.2019-0187
• Research & Development • Previous Articles Next Articles
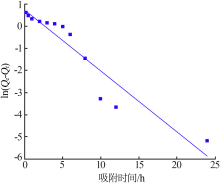
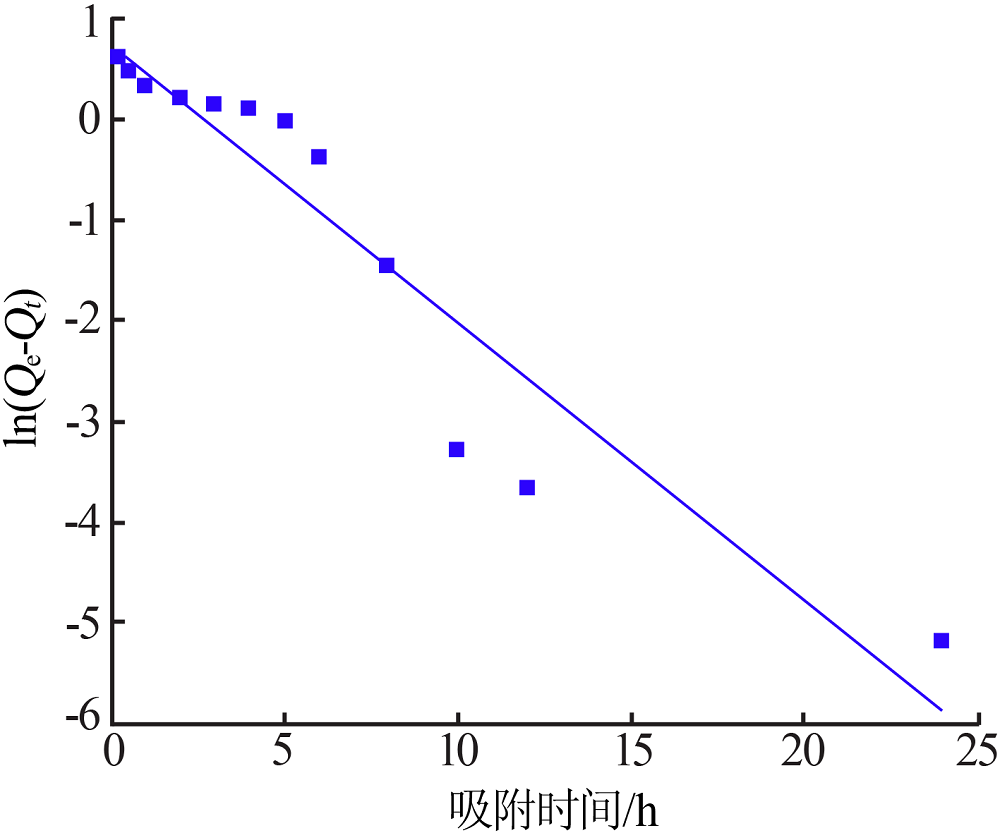
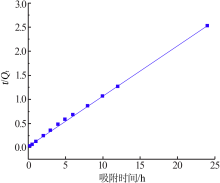
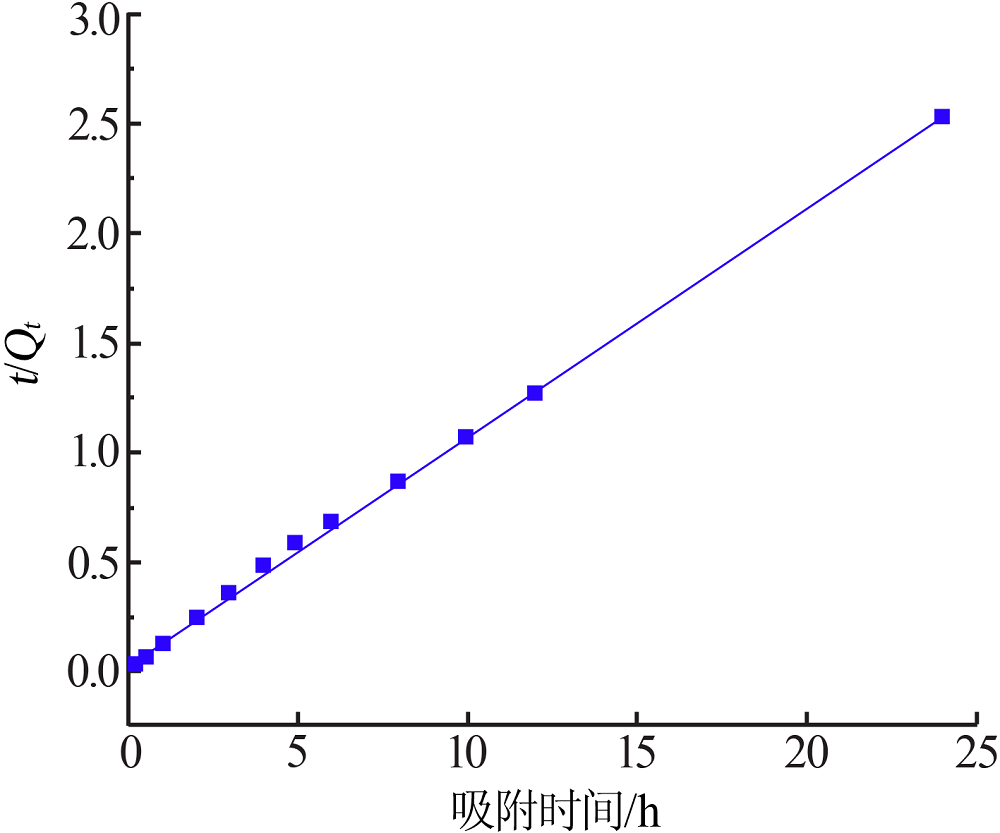
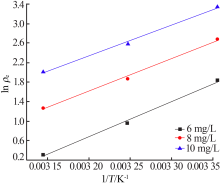
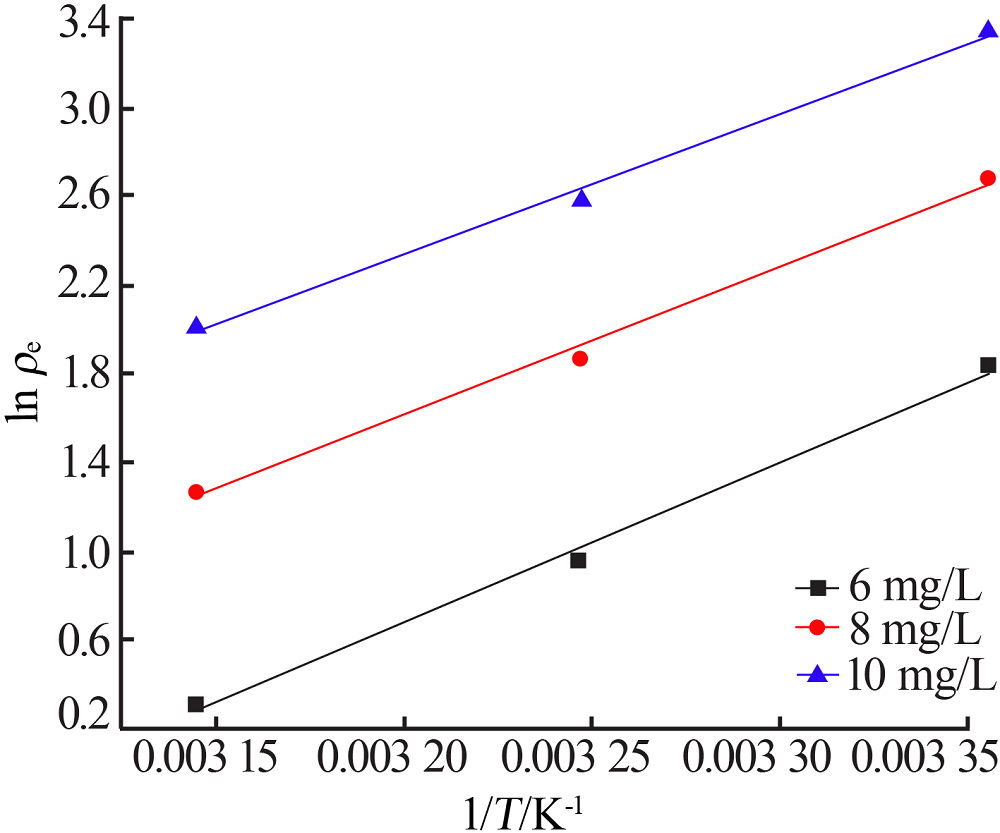
Research on adsorption of Ni 2+ by carbonated hydroxyapatite/potash feldspar composite materials
Zhang Ting,Zhu Huixia,Han Cong,Sun Lina,Li Ling( )
)
- College of Chemistry and Chemical Engineering,Xinjiang University,Urumqi 830046,China
-
Received:2019-08-18Online:2020-02-10Published:2020-02-26 -
Contact:Li Ling E-mail:llnwzd2@163.com
CLC Number:
Cite this article
Zhang Ting,Zhu Huixia,Han Cong,Sun Lina,Li Ling. Research on adsorption of Ni 2+ by carbonated hydroxyapatite/potash feldspar composite materials[J]. Inorganic Chemicals Industry, 2020, 52(2): 30-34.
share this article
| [1] | Farooq U, Kozinski J A, Khan M A , et al. Biosorption of heavy metal ions using wheat based biosorbents-A review of the recent literat-ure[J]. Bioresource Technology, 2010,101:5043-5053. |
| [2] | 刘羽, 胥焕岩, 黄志良 , 等. 羟基磷灰石吸附水溶液Cd 2+的影响因素的研究 [J]. 岩石矿物学杂志, 2001,20(4):583-586. |
| [3] | 钱程, 张卫民 . 纳米HAP复合型材料吸附水中镍离子性能[J]. 无机盐工业, 2019,51(2):45-49. |
| [4] | Yang Z M, Fang Z Q, Tang P E , et al. In situ remediation and phyto-toxiticy assessment of lead-contaminated soil by biochar-supported nHAP[J]. Journal of Environmental Management, 2016,182:247-251. |
| [5] | He M, Shi H, Zhao X Y , et al. Immobilization of Pb and Cd in con-taminated soil using nano-crystallite hydroxyapatite[J]. Procedia Environmental Sciences, 2013,18:657-665. |
| [6] | 车丽诗, 袁毳, 雷鸣 , 等. 改性活性炭和改性沸石去除电解锰渣淋洗液中锰离子的研究[J]. 水处理技术, 2017,43(7):76-80. |
| [7] | Zhou Y T, Nie H L, Branford-White C , et al. Removal of Cu 2+ from aqueous solution by chitosan-coated magnetic nanoparticles modi-fied with aketoglutaric acid[J]. Journal of Colloid and Interface Science, 2009,330:29-37. |
| [8] | Tseng R L, Wu F C . Analyzing liquid-solid phase countercurrent two-and three-stage adsorption process with the Freundlich equation[J]. Journal of Hazardous Materials, 2009,162(1):237-248. |
| [9] | Yao Q, Xie J, Liu J , et al. Adsorption of lead ions using a modified lignin hydrogel[J]. Journal of Polymer Research, 2014,21(6):465-479. |
| [10] | 马叶, 刘斌, 孙楠 , 等. 改性活性炭对水中铬离子(Ⅵ)的吸附性能[J]. 环境工程学报, 2014,8(7):2672-2676. |
| [11] | 蓝梅, 王丽君, 张会宁 , 等. 柠檬酸改性活性炭对水中Cr(Ⅵ)的吸附性能研究[J]. 水处理技术, 2018,44(9):80-85. |
| [12] | Alshameri A, Yan C, Al-Ani Y , et al. An investigation into the ad-sorption removal of ammonium by salt activated Chinese(Hulaodu)natural zeolite:Kinetics,isotherms,and thermodynamics[J]. Journal of the Taiwan Institute of Chemical Engineers, 2014,45(2):554-564. |
| [13] | 马宏飞, 李薇, 韩秋菊 , 等. 废茶渣对Cr(Ⅵ)的等温吸附模型研究[J]. 科学技术与工程, 2012,12(25):323-325. |
| [14] | Irani M, Amjadi M, Mousavian M A . Comparative study of lead so-rption onto natural perlite,dolomite and diatomite[J]. Chemical Engineering Journal, 2011,178(24):317-323. |
| [15] | 付杰, 李燕虎, 叶长燊 , 等. DMF在大孔吸附树脂上的吸附热力学及动力学研究[J]. 环境科学学报, 2012,32(3):639-644. |
| [16] | Gómez del Río J A, Morando P J, Cicerone D S . Natural materials for treatment of industrial effluents:Comparative study of the re-tention of Cd,Zn and Co by calcite and hydroxyapatite.Part Ι:Ba-tch experiments[J]. Journal of Environmental Management, 2004,71:169-177. |
| [1] | Qingqing Jin,Xiaoyi Liang,Jia′nan Zhang,Xiaolong Zhou. Study on adsorption performance of modified spherical activated carbon for ammonia [J]. Inorganic Chemicals Industry, 2021, 54(4): 61-66. |
| [2] | Yang Dian,Wang Fang,Wan Ye,Sun Qiang,Zhang Zheng,Shi Qinghe. Research progress on separation process of methyl dichlorosilane from trichlorsilane [J]. Inorganic Chemicals Industry, 2021, 53(3): 30-33. |
| [3] | Xu Naicai,Shi Dandan. Preparation of manganese dioxide nanomaterials with different crystalline and their adsorption properties to methylene blue [J]. Inorganic Chemicals Industry, 2021, 53(3): 44-47. |
| [4] | Wu Zhaomin,Yang Lin,Wang Xinlong,Zhuang Haibo,Ye Runzhou,Wang Ye,Li Yaoji. Study on process of dynamic ion-exchange for etching waste acid containing aluminum in electronic industry [J]. Inorganic Chemicals Industry, 2021, 53(2): 61-65. |
| [5] | Han Cong,Sun Lina,Zhang Ting,Zhu Huixia,Li Ling. Study on adsorption of phenol in wastewater by loading modified potassium feldspar [J]. Inorganic Chemicals Industry, 2021, 53(2): 71-76. |
| [6] | Liu Darui,Zhu Dandan. Study on adsorption and desorption of phosphate by coal gasification slag [J]. Inorganic Chemicals Industry, 2021, 53(2): 84-87. |
| [7] | Zhuang Haibo,Yang Lin,Deng Qiang,Ye Runzhou,Wu Zhaomin. Study on dynamic adsorption of Mn 2+ from phosphoric acid by ion exchange technology [J]. Inorganic Chemicals Industry, 2021, 53(1): 18-23. |
| [8] | Liu Yuanhui,Tang Xiaona,Xie Lei,Jiang Xiaopeng,Zhang Yunbo. Investigation progress of ageing process of plaster of paris [J]. Inorganic Chemicals Industry, 2020, 52(9): 15-20. |
| [9] | Tang Qinyuan,An Yan,Sun Qi,Guo Xingqiang,Li Qingqing,Guo Bingchen. Amino-functionalized Al-magadiite for Hg2+ adsorption [J]. Inorganic Chemicals Industry, 2020, 52(8): 40-45. |
| [10] | Li Haichao,Ji Qingsong,Zhang Jingjing,Yang Siqiao. Purification of industrial potassium chloride from salt lake by activated pig bone biochar [J]. Inorganic Chemicals Industry, 2020, 52(8): 76-77. |
| [11] | Gu Junjie,Li Zengrong,Tang Faman,Zhang Xu,Hou Miaomiao,Wang Xiaohu. Study on continuous ion exchange process applied in lithium extraction from salt lake brine with adsorption method [J]. Inorganic Chemicals Industry, 2020, 52(7): 46-51. |
| [12] | Xu Naicai,Shi Dandan,Li Sixia. Research on structure regulation and fluorine removal property of mesoporous alumina [J]. Inorganic Chemicals Industry, 2020, 52(6): 20-23. |
| [13] | Hu Dongwan,Ma Zhanling,Ma Xiao,Li Jianrong. Preparation of modified Fe3O4-pectin magnetic microspheres and its adsorption property for Pb 2+ [J]. Inorganic Chemicals Industry, 2020, 52(6): 24-29. |
| [14] | Xu Naicai,Li Sixia,Cao Jiajia,Yang Gangshenga. Research on doping modification and adsorption performance of manganese oxides lithium ion sieve [J]. Inorganic Chemicals Industry, 2020, 52(4): 37-41. |
| [15] | He Yugang,Wang Fang,Sun Qiang,Zhang Xiaowei,Shi Qinghe,Fu Qiang. Effect of adsorption device position on boron and phosphorus removal in polycrystalline silicon [J]. Inorganic Chemicals Industry, 2020, 52(3): 72-74. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|