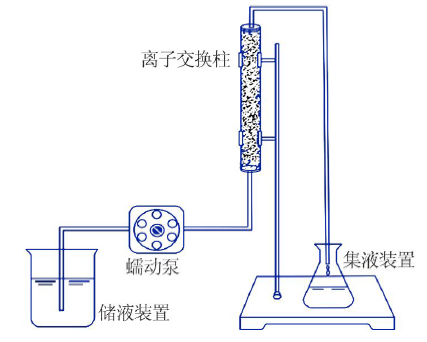
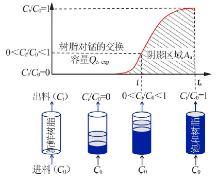
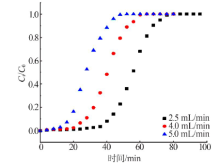
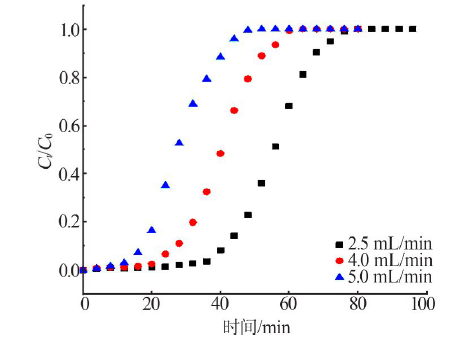
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (1): 18-23.doi: 10.11962/1006-4990.2020-0096
Previous Articles Next Articles
Study on dynamic adsorption of Mn 2+ from phosphoric acid by ion exchange technology
Zhuang Haibo( ),Yang Lin(
),Yang Lin( ),Deng Qiang,Ye Runzhou,Wu Zhaomin
),Deng Qiang,Ye Runzhou,Wu Zhaomin
- School of Chemical Engineering,Sichuan University,Chengdu 610065,China
-
Received:2020-07-18Online:2021-01-10Published:2021-01-08 -
Contact:Yang Lin E-mail:1260387626@qq.com;18980632893@163.com
CLC Number:
Cite this article
Zhuang Haibo,Yang Lin,Deng Qiang,Ye Runzhou,Wu Zhaomin. Study on dynamic adsorption of Mn 2+ from phosphoric acid by ion exchange technology[J]. Inorganic Chemicals Industry, 2021, 53(1): 18-23.
share this article
"
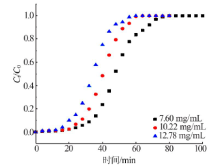
| C0 / (mg·mL-1) | q/ (mL·min-1) | T/ ℃ | m/ g | Qe,exp / (mg·g-1) | Qe,Th / (mg·g-1) | 偏差/ % | kTh / (mL·min-1·mg-1) | R2 |
|---|---|---|---|---|---|---|---|---|
| 10.22 | 2.5 | 20 | 22.50 | 56.50 | 60.71 | 7.45 | 0.013 16 | 0.953 2 |
| 10.22 | 4.0 | 20 | 22.52 | 65.69 | 70.06 | 6.66 | 0.016 40 | 0.962 0 |
| 10.22 | 5.0 | 20 | 22.47 | 57.94 | 63.50 | 9.61 | 0.021 24 | 0.985 0 |
| 7.60 | 4.0 | 20 | 22.52 | 56.63 | 64.16 | 13.31 | 0.017 90 | 0.992 7 |
| 10.22 | 4.0 | 20 | 22.52 | 65.69 | 70.06 | 6.66 | 0.016 40 | 0.962 0 |
| 12.78 | 4.0 | 20 | 22.57 | 70.49 | 75.84 | 7.59 | 0.013 66 | 0.991 2 |
| 10.22 | 4.0 | 20 | 22.52 | 65.69 | 70.06 | 6.65 | 0.016 40 | 0.962 0 |
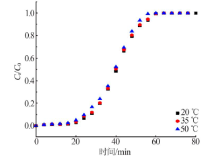
| 10.22 | 4.0 | 35 | 22.56 | 64.76 | 68.53 | 5.82 | 0.016 45 | 0.958 7 |
| 10.22 | 4.0 | 50 | 22.42 | 63.21 | 66.32 | 4.92 | 0.016 57 | 0.951 8 |
"
| C0 / (mg·mL-1) | q/ (mL·min-1) | T/ ℃ | m/ g | τexp / min | τcal / min | 偏差/ % | kYN / min-1 | R2 |
|---|---|---|---|---|---|---|---|---|
| 10.22 | 2.5 | 20 | 22.50 | 57.41 | 53.47 | 6.86 | 0.134 5 | 0.985 0 |
| 10.22 | 4.0 | 20 | 22.52 | 42.31 | 38.60 | 8.77 | 0.167 6 | 0.962 0 |
| 10.22 | 5.0 | 20 | 22.47 | 30.66 | 27.93 | 8.91 | 0.217 0 | 0.953 2 |
| 7.60 | 4.0 | 20 | 22.52 | 50.38 | 47.53 | 5.66 | 0.136 0 | 0.992 7 |
| 10.22 | 4.0 | 20 | 22.52 | 42.54 | 38.60 | 9.26 | 0.167 6 | 0.962 0 |
| 12.78 | 4.0 | 20 | 22.57 | 36.81 | 33.48 | 9.04 | 0.174 6 | 0.991 2 |
| 10.22 | 4.0 | 20 | 22.52 | 42.54 | 38.60 | 9.26 | 0.167 6 | 0.962 0 |
| 10.22 | 4.0 | 35 | 22.56 | 42.23 | 37.82 | 10.44 | 0.168 1 | 0.958 7 |
| 10.22 | 4.0 | 50 | 22.42 | 41.68 | 36.38 | 12.73 | 0.169 3 | 0.951 8 |
"
| C0 / (mg·mL-1) | q/ (mL·min-1) | T/℃ | m/g | Qe,exp/ (mg·g-1) | Qe,Yan/ (mg·g-1) | 偏差/% | a | b/mL | R2 |
|---|---|---|---|---|---|---|---|---|---|
| 10.22 | 2.5 | 20 | 22.50 | 56.50 | 62.43 | 10.48 | 9.456 | 137.45 | 0.998 1 |
| 10.22 | 4.0 | 20 | 22.52 | 65.69 | 72.04 | 9.67 | 7.187 | 158.76 | 0.997 6 |
| 10.22 | 5.0 | 20 | 22.47 | 57.94 | 61.95 | 6.93 | 5.391 | 136.23 | 0.998 3 |
| 7.60 | 4.0 | 20 | 22.52 | 56.62 | 63.85 | 12.76 | 7.023 | 189.19 | 0.998 6 |
| 10.22 | 4.0 | 20 | 22.52 | 65.69 | 72.04 | 9.67 | 7.187 | 158.76 | 0.997 6 |
| 12.78 | 4.0 | 20 | 22.57 | 70.49 | 76.02 | 7.84 | 6.266 | 134.25 | 0.996 9 |
| 10.22 | 4.0 | 20 | 22.52 | 65.69 | 72.04 | 9.66 | 7.19 | 158.76 | 0.997 6 |
| 10.22 | 4.0 | 35 | 22.56 | 64.76 | 71.23 | 10.00 | 7.13 | 157.26 | 0.996 8 |
| 10.22 | 4.0 | 50 | 22.42 | 63.21 | 70.11 | 10.91 | 7.03 | 153.81 | 0.993 4 |
| [1] |
Kouzbour S, Gourich B, Gros F, et al. Comparative analysis of indus-trial processes for cadmium removal from phosphoric acid:A revi-ew[J]. Hydrometallurgy, 2019,188:222-247.
doi: 10.1016/j.hydromet.2019.06.014 |
| [2] | 马超, 吴元欣, 金放, 等. 磷酸的工业生产研究现状与展望[J]. 化学工程, 2013,41(6):74-78. |
| [3] | 王同永. 湿法磷酸净化技术应用研究[J]. 山东化工, 2018,47(12):122-123. |
| [4] | 张亚明, 李文超, 王海军. 我国磷矿资源开发利用现状[J]. 化工矿物与加工, 2020,49(6):43-46. |
| [5] | 罗惠华, 刘连坤, 朱道鹏, 等. 黄麦岭磷矿除镁降锰选矿试验研究[J]. 中国非金属矿工业导刊, 2017(1):17-20. |
| [6] | 黄懿, 张志业, 王辛龙, 等. 溶剂萃取法脱除湿法磷酸中锰离子的实验研究[J]. 磷肥与复肥, 2018,33(4):5-7. |
| [7] | Leng X, Zhong Y, Xu D, et al. Mechanism and kinetics study on removal of iron from phosphoric acid by cation exchange resin[J]. Chin.J.Chem.Eng., 2019,27(5):1050-1057. |
| [8] | Tang C, Qiu Y, Wang Y, et al. Kinetic studies on Al3+ removal from phosphoric acid by cation exchange resin[J]. Can.J.Chem.Eng., 2018,96(4):944-954. |
| [9] |
Abdel-Ghafar H M, Abdel-Aal E A, Ibrahim M A M, et al. Purifi-cation of high iron wet-process phosphoric acid via oxalate precipi-tation method[J]. Hydrometallurgy, 2019,184:1-8.
doi: 10.1016/j.hydromet.2018.12.011 |
| [10] |
Jin Y, Ma Y, Weng Y, et al. Solvent extraction of Fe3+ from the hy-drochloric acid route phosphoric acid by D2EHPA in kerosene[J]. Journal of Industrial and Engineering Chemistry, 2014,20(5):3446-3452.
doi: 10.1016/j.jiec.2013.12.033 |
| [11] | Dang L, Wei H, Zhu Z, et al. The influence of impurities on phosp-horic acid hemihydrate crystallization[J]. J.Cryst.Growth, 2007,307(1):104-111. |
| [12] | 杨帆, 胡方峰, 陈金芳. 十二烷基苯磺酸乳化液膜法净化湿法磷酸除镁[J]. 云南化工, 2008(1):24-29. |
| [13] | 刘安荣, 李敬, 王振杰, 等. 离子交换法脱除湿法磷酸中铁、铝杂质的研究[J]. 化工矿物与加工, 2019,48(5):57-59. |
| [14] | 熊祥祖, 王威, 李志保, 等. 离子交换树脂脱除湿法磷酸中金属杂质的研究[J]. 武汉工程大学学报, 2009,31(7):26-29. |
| [15] | 杨政琦, 周学克, 阮云刚, 等. 离子交换树脂脱除湿法磷酸中的铝和镁的研究[J]. 磷肥与复肥, 2016,31(2):6-7,10. |
| [16] | 龚浩, 郭劲松, 方芳, 等. 改性陶粒对水中卡马西平去除的动态吸附实验及模型[J]. 环境工程学报, 2016,10(7):3573-3579. |
| [17] | 李亚娟, 赵传起, 杨悦锁, 等. 石墨烯基铁氧化物对水体中草甘膦的动态吸附性能及模型[J]. 化工学报, 2018,69(9):3944-3953. |
| [18] | 袁纯怡, 孙玉柱, 杨颖, 等. D301树脂动态吸附溴离子过程探究及模型拟合[J]. 过程工程学报, 2020(6):655-666. |
| [1] | Yin Wei,Huang Jin,Xie Tian,He Bingbing. Experimental study on high efficiency decomposition of phosphate rock by phosphoric acid [J]. Inorganic Chemicals Industry, 2020, 52(9): 34-36. |
| [2] | Wang Xiaoxiong,Zhang Jiangang,Zhang Wenjing,Chen Ye. Removal of calcium chloride from crude phosphoric acid by hydrochloric acid method [J]. Inorganic Chemicals Industry, 2020, 52(8): 17-19. |
| [3] | Wang Xiheng,Sun Wenzhe. Research progress on fluorine recovery technology in wet process phosphoric acid [J]. Inorganic Chemicals Industry, 2020, 52(8): 25-29. |
| [4] | Gu Junjie,Li Zengrong,Tang Faman,Zhang Xu,Hou Miaomiao,Wang Xiaohu. Study on continuous ion exchange process applied in lithium extraction from salt lake brine with adsorption method [J]. Inorganic Chemicals Industry, 2020, 52(7): 46-51. |
| [5] | Xu Xinfang,Zhou Xiaoping,Liu Zhengfeng,Li Lei,Chen Hu,Li Changming. Study on causticization and purification of salt lake lithium ore and recovery of fluorine-containing lithium carbonate [J]. Inorganic Chemicals Industry, 2020, 52(7): 62-65. |
| [6] | Chen Yong,Yang Sanke,Xie Tian,Tao Shaocheng,Long Qinglan,Liu Xu. Research on preparation process of water-soluble ammonium polyphosphate with high polymerization rate [J]. Inorganic Chemicals Industry, 2020, 52(5): 50-52. |
| [7] | Zhang Xiaoxia. Study on process of producing lithium phosphate by low-concentration lithium-containing waste liquid [J]. Inorganic Chemicals Industry, 2020, 52(5): 68-70. |
| [8] | Peng Wenbo,Li Tengfei,Bai Zuguo,Chen Daokang,Yun Jianjun. Application of membrane technology in resource recovery of cobalt-containing saline wastewater [J]. Inorganic Chemicals Industry, 2020, 52(5): 65-67. |
| [9] | Wang Baoming,Lü Zhonghao,Li Huiyong,Liu Yong,Hua Quanxian,Tang Jianwei. Research progress on preparation of urea phosphate with wet-process phosphoric acid [J]. Inorganic Chemicals Industry, 2020, 52(11): 1-5. |
| [10] | Wang Zhengwei,Li Xiaobo,Liang Huili,Li Peiying. Analysis and calculation of heat balance and heat utilization efficiency in hydration tower of thermal phosphoric acid [J]. Inorganic Chemicals Industry, 2020, 52(11): 95-98. |
| [11] | Zhou Zhenjun,Zhang Hao,Luo Weihua,Xu Peijun,Cong Peiliang. Effect of hydrothermal ion exchange time on remodeling of leucite by cesium ion [J]. Inorganic Chemicals Industry, 2019, 51(9): 17-20. |
| [12] | Chang Jun,Jia Fukang,Hu Chengshan,Ye Qianxu. Adsorption of manganese ion by zeolite synthesized from electrolytic manganese residue [J]. Inorganic Chemicals Industry, 2019, 51(9): 61-66. |
| [13] | Zhao Chuang1,Su Wenli2,Zang Jiazhong1,Sun Lei2,Fan Jingxin1,Ma Yuming1,Li Ben1,Li Bin1,Yu Haibin1. Separation technology of alkane/olefin of two AgX modified adsorbents [J]. Inorganic Chemicals Industry, 2019, 51(7): 100-103. |
| [14] | Wang Ben,Chen Mengmeng,Zhang Fan. Research progress on purification technology of hydrogen peroxide [J]. Inorganic Chemicals Industry, 2019, 51(6): 1-4. |
| [15] | Sun Fengjuan,Liu Wanchao,Yan Kun,E Yishuai. Removal of chloride ion from desulfurization wastewater by ion-exchange resin [J]. Inorganic Chemicals Industry, 2019, 51(6): 45-48. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|