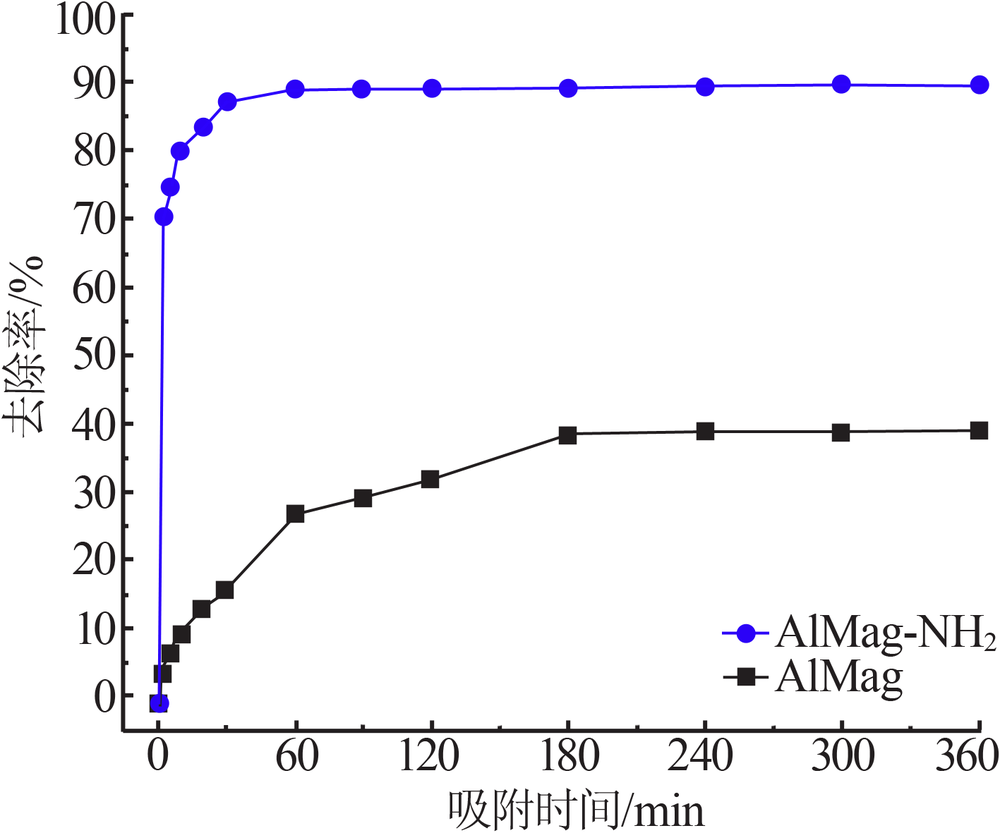
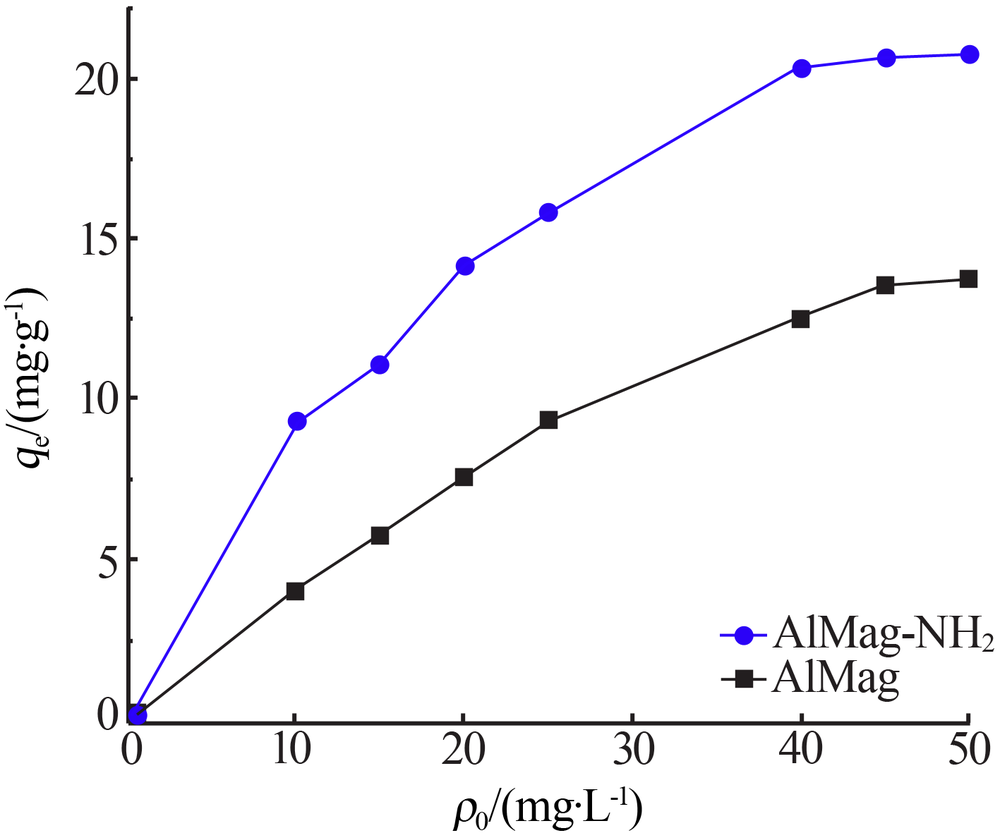
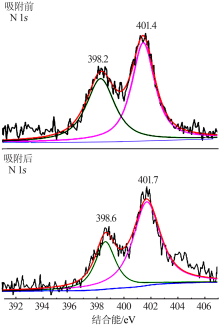
| [1] |
Das S, Arnab S, Gautam G, et al. Clay-based nanocomposites as recyclable adsorbent toward Hg(Ⅱ) capture: Experimental and theoretical understanding[J]. ACS Omega, 2018,3:6283-6292.
doi: 10.1021/acsomega.8b00789
pmid: 31458810
|
| [2] |
Krabbenhoft D P, Sunderland E M. Global change and mercury[J]. Science, 2013,341(6153):1457-1458.
doi: 10.1126/science.1242838
pmid: 24072910
|
| [3] |
朱慧霞, 张婷, 韩琮, 等. 钾长石负载二氧化锰对Ni2+的吸附性能研究 [J]. 无机盐工业, 2019,52(1):44-48.
|
| [4] |
陈济美, 龚关, 赵连强, 等. 膨胀土阳离子交换容量的测定[J]. 岩矿测试, 2000,19(2):152-154.
|
| [5] |
Guerra D L, Pinto A A, Souza J A, et al. Kinetic and thermodynamicuranyl(Ⅱ) adsorption process into modified Na-magadiite and Nakanemite[J]. Journal of Hazardous Materials, 2009,166(2/3):1550-1555.
|
| [6] |
Benkhatou S, Djelad A, Sassi M, et al. Lead(Ⅱ) removal from aqueous solutions by organic thiourea derivatives intercalated magadiite[J]. Desalination and Water Treatment, 2015,57(20):9383-9395.
|
| [7] |
戈明亮, 杜明艺, 王雁武. 介孔材料麦羟硅钠石吸附Pb2+的行为及机理 [J]. 硅酸盐学报, 2017,45(1):37-45.
|
| [8] |
Ahmed K, Khan A J, Yaminc M, et al. Fabrication of layered Al-silicate magadiites for the removal of reactive dye from textile effluents[J]. Desalination and Water Treatment, 2018,104:159-168.
|
| [9] |
Bi Y F, Blanchard J, Lambert J F. Role of the Al source in the synjournal of aluminum magadiite[J]. Applied Surface Science, 2012,57:71-78.
|
| [10] |
Kooli F, Mianhui L, Alshahateet S, et al. Characterization and thermal stability properties of intercalated Na-magadiite with cetyltrimethylammonium(C16TMA) surfactants[J]. Journal of Physics and Chemistry of Solids, 2006,67:926-931.
|
| [11] |
Isoda K, Kuroda K, Ogawa M. Interlamellar grafting of γ-methacry loxypropylsilyl groups on magadiite and copolymerization with methyl methacrylate[J]. Chemistry of Materials, 2000,12:1702-1707.
|
| [12] |
Superti G B, Oliveira E C, Pastore H O. Aluminum magadiite:An acid solid layered material[J]. Chemistry of Materials, 2007,19:4300-4315.
|
| [13] |
Park K W, Jung J H, Seo H J, et al. Mesoporous silica-pillared kenyaite and magadiite as catalytic support for partial oxidation of methane[J]. Microporous and Mesoporous Materials, 2009,12:219-225.
|
| [14] |
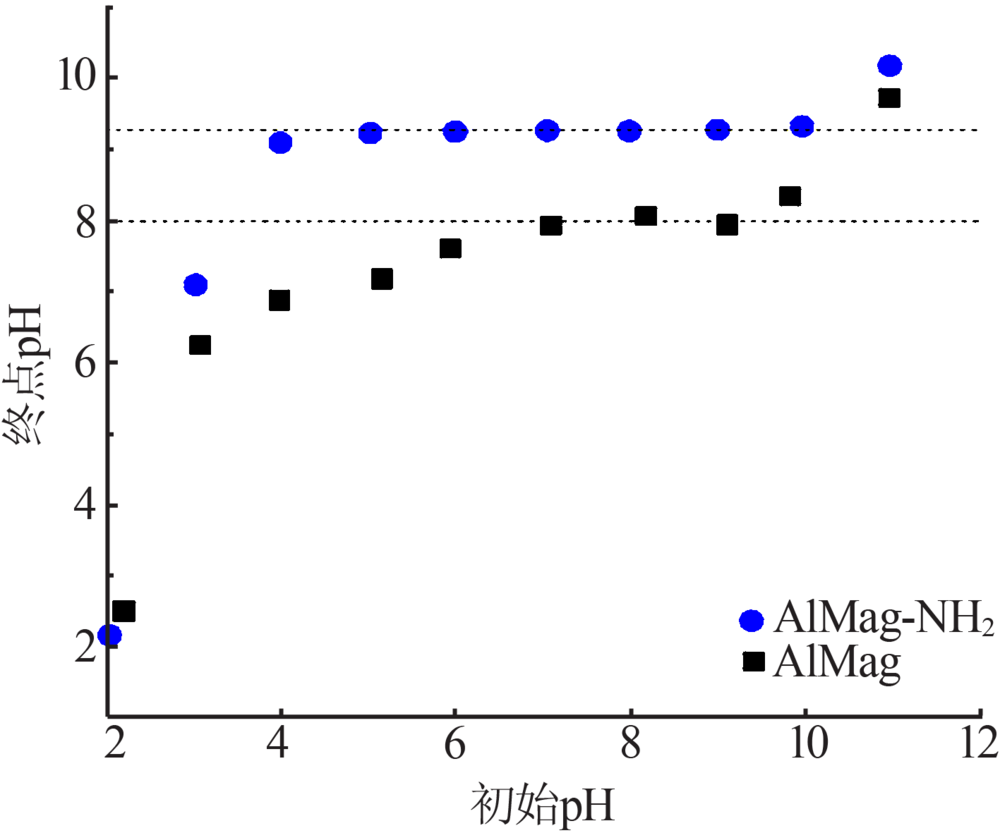
Bourikas K, Vakros J, Kordulis C, et al. Potentiometric mass titrations: experimental and theoretical establishment of a new technique for determining the point of zero charge(pzc) of metal(hydr)oxi-des[J]. Journal of Physical Chemistry B, 2003,107:9441-9451.
|
| [15] |
Chandra V, Kim K S. Highly selective adsorption of Hg2+ by a polypyrrole-reduced grapheme oxide composite [J]. Chemical Communications, 2011,47(13):3942-3944.
doi: 10.1039/c1cc00005e
pmid: 21350767
|
| [16] |
Xu H, Xu D C, Wang Y F. Natural indices for the chemical hardness/softness of metal cations and ligands[J]. ACS Omega, 2017,2:7185-7193.
doi: 10.1021/acsomega.7b01039
pmid: 31457297
|
| [17] |
Tran L, Wu P X, Zhu Y J, et al. Comparative study of Hg(Ⅱ) adsorption by thiol-and hydroxyl-containing bifunctional montmorillonite and vermiculite[J]. Applied Surface Science, 2015,356:91-101.
doi: 10.1016/j.apsusc.2015.08.038
|
| [18] |
陈理想, 吴平霄, 杨林, 等. 有机改性蛭石的特性及其对Hg2+吸附性能的研究 [J]. 环境科学学报, 2015,35(4):1054-1060.
|
| [19] |
Cui H, Qian Y, Li Q, et al.Adsorption of aqueous Hg(Ⅱ) by a polyaniline/attapulgite composite[J].Chemical Engineering Journal, 2012,211/212:216-223.
doi: 10.1016/j.cej.2012.09.057
|
| [20] |
Sun J, Chen D, Ge M, et al.Selective adsorption of Hg(Ⅱ) by γ-radiation synthesized silica-graft-vinyl imidazole adsorbent[J]. Journal of Hazardous Materials, 2013,244/245:94-101.
|
 ),An Yan1(
),An Yan1( ),Sun Qi1,2,Guo Xingqiang3,Li Qingqing1,Guo Bingchen1
),Sun Qi1,2,Guo Xingqiang3,Li Qingqing1,Guo Bingchen1