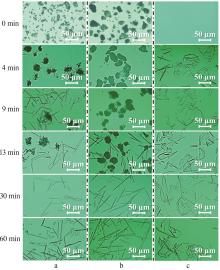
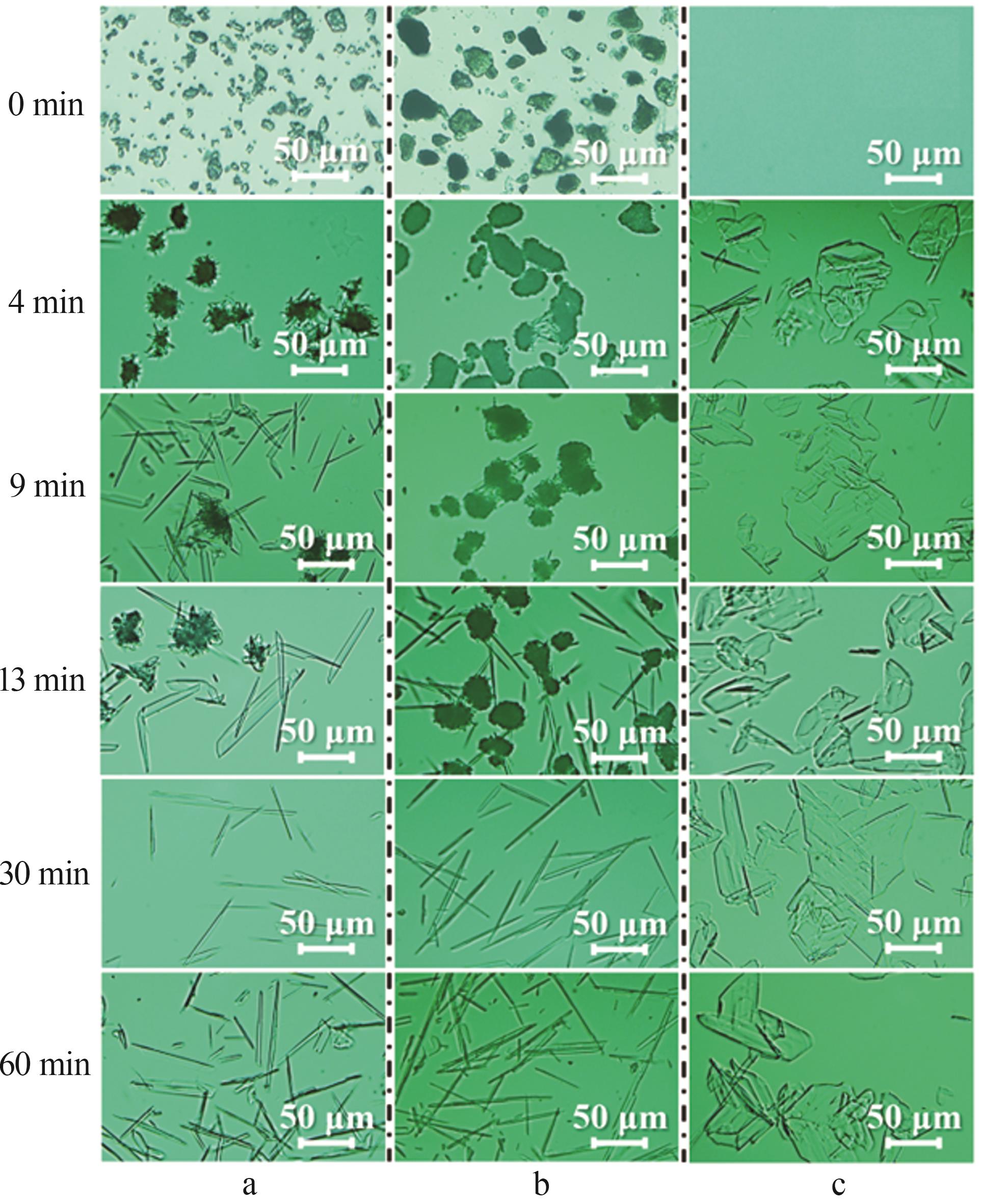
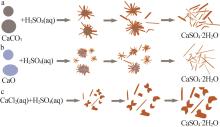
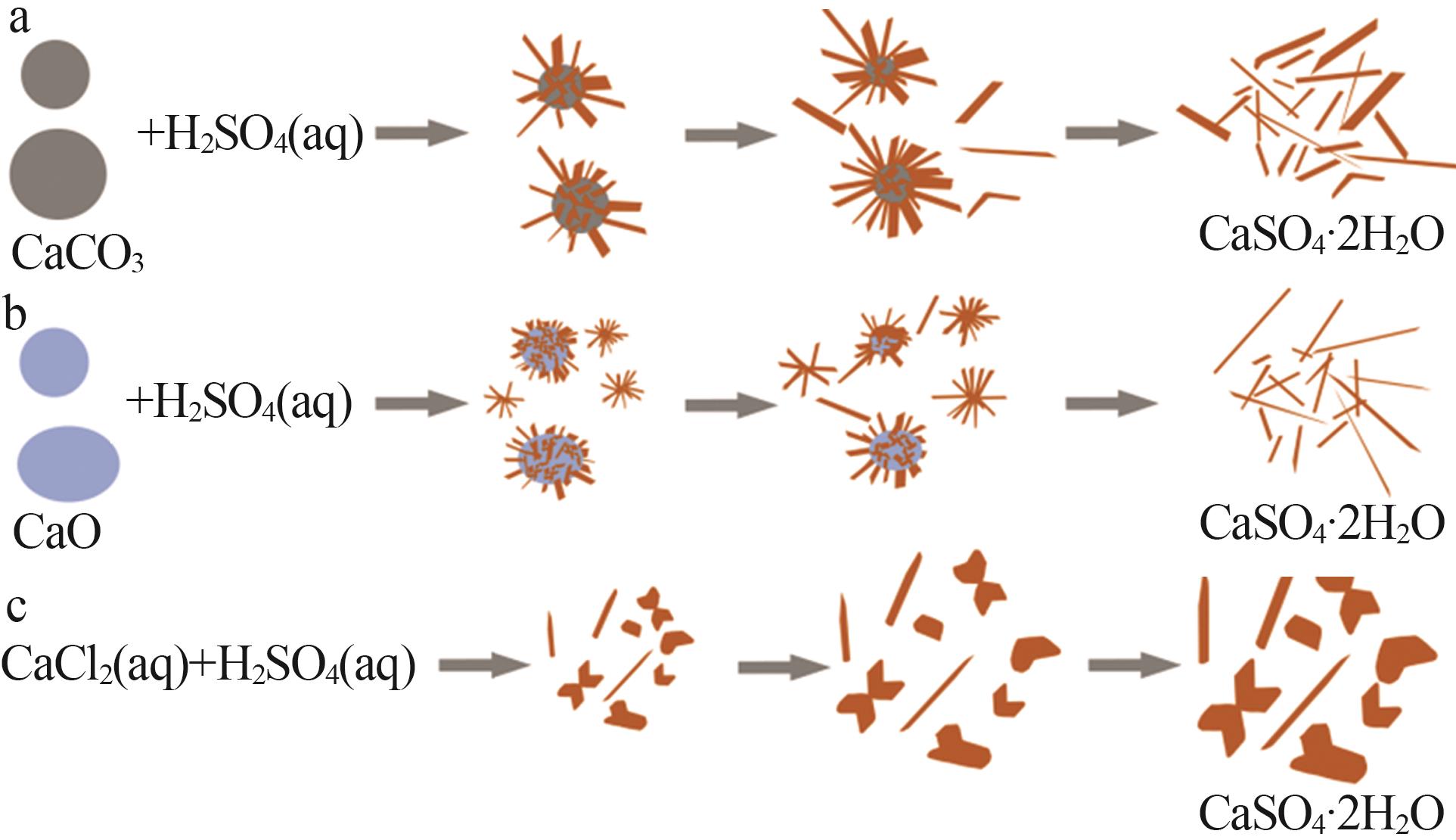
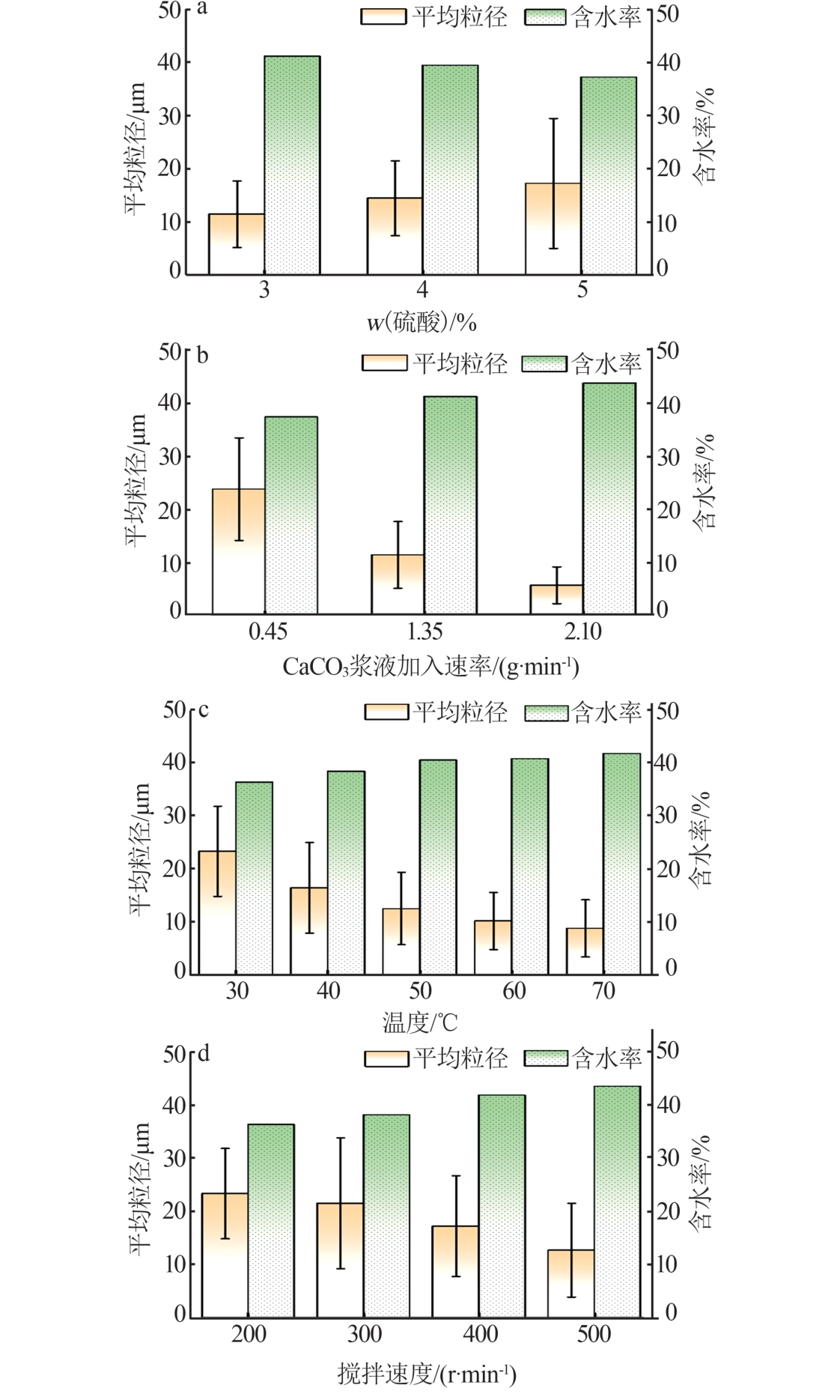
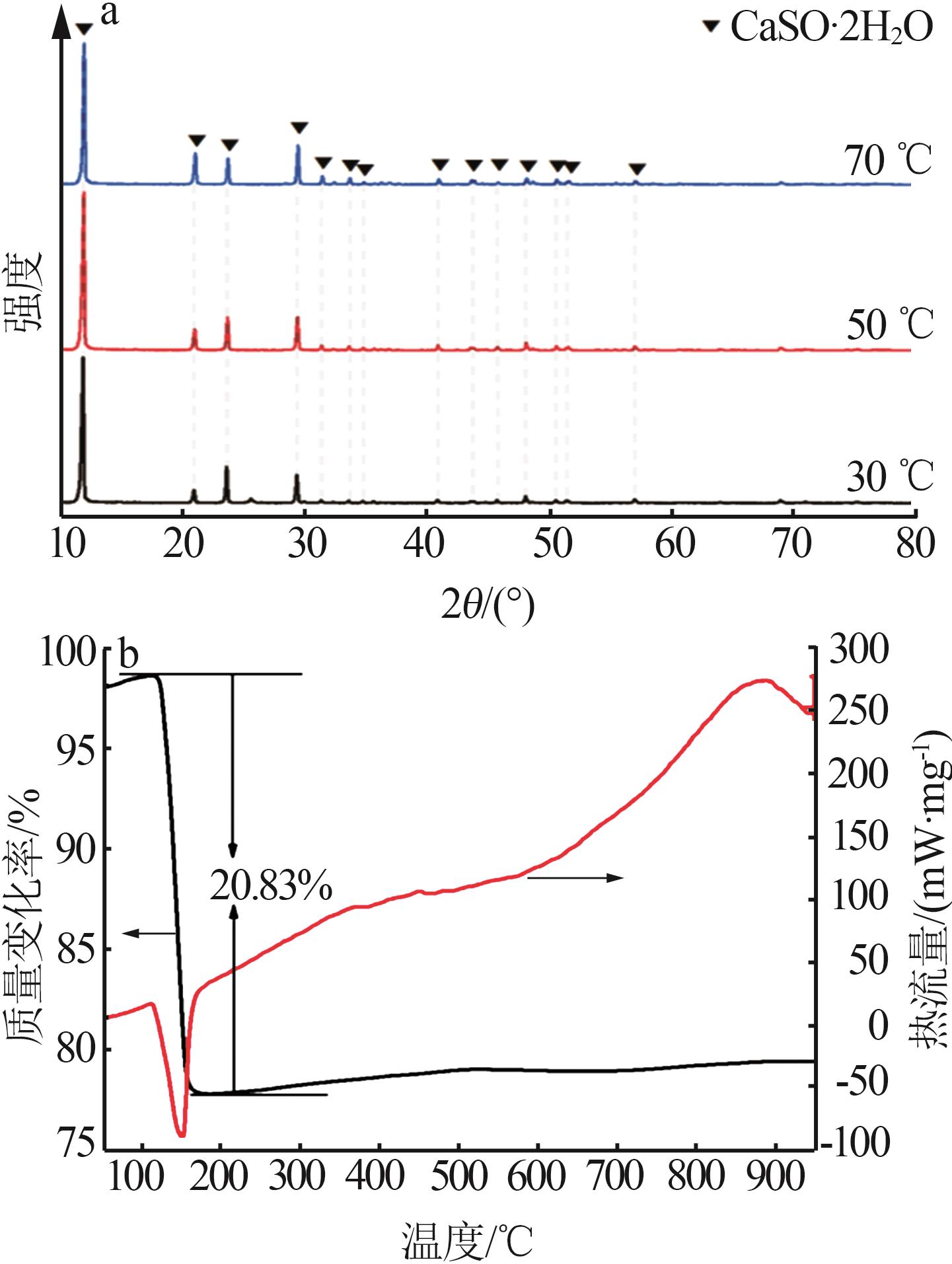
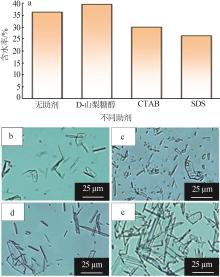
| [1] |
孙启,黄松涛,杨丽梅,等.工业废酸中硫酸的萃取分离与回收研究现状[J].稀有金属,2022,46(5):652-664.
|
|
SUN Qi, HUANG Songtao, YANG Limei,et al.Separation and recovery of sulfuric acid from industrial spent acid by solvent extraction:A review[J].Chinese Journal of Rare Metals,2022,46(5):652-664.
|
| [2] |
纪罗军.我国工业废硫酸资源化利用技术进展[J].硫酸工业,2017(3):4-10.
|
|
JI Luojun.Technical progress of industrial waste sulphuric acid utilization in China[J].Sulphuric Acid Industry,2017(3):4-10.
|
| [3] |
周强,李庆,毛三万,等.钛白粉废酸的处理方法与研究[J].清洗世界,2023,39(1):63-65.
|
|
ZHOU Qiang, LI Qing, MAO Sanwan,et al.Treatment method and research of titanium dioxide waste acid[J].Cleaning World,2023,39(1):63-65.
|
| [4] |
RABIZADEH T, STAWSKI T M, MORGAN D J,et al.The effects of inorganic additives on the nucleation and growth kinetics of calcium sulfate dihydrate crystals[J].Crystal Growth & Design,2017,17(2):582-589.
|
| [5] |
DENG Licong, ZHANG Yifei, CHEN Fangfang,et al.Reactive crystallization of calcium sulfate dihydrate from acidic wastewater and lime[J].Chinese Journal of Chemical Engineering,2013,32(11):1303-1312.
|
| [6] |
易家祥,谢卫苹,伏天兵.电石渣中和废稀酸制备高强石膏应用分析[J].环境与发展,2019,31(5):146-147.
|
|
YI Jiaxiang, XIE Weiping, FU Tianbing.Application analysis of high strength gypsum prepared by neutralizing carbide slag and waste dilute acid[J].Environment and Development,2019,31(5):146-147.
|
| [7] |
秦颖,刘新庄,桑海风.工业副产石膏在建材领域的资源化利用现状及展望[J].中国建材科技,2023,32(5):23-25.
|
|
QIN Ying, LIU Xinzhuang, SANG Haifeng.Current situation and prospect of resource utilization of industrial by-product gypsum in the field of building materials[J].China Building Materials Science & Technology,2023,32(5):23-25.
|
| [8] |
张彦锋,马永亮,王正源,等.石灰石-石膏法烟气脱硫系统处理废稀硫酸[J].化工环保,2022,42(1):114-118.
|
|
ZHANG Yanfeng, MA Yongliang, WANG Zhengyuan,et al.Treatment of spent dilute sulfuric acid in fuel gas desulfurization system by using limestone-gypsum method[J].Environmental Protection of Chemical Industry,2022,42(1):114-118.
|
| [9] |
JU Jinrong, FENG Yali, LI Haoran,et al.A novel approach for separation and recovery of titanium,scandium,iron from acidic wastewater and red gypsum utilization[J].Mining,Metallurgy & Exploration,2022,39(3):1297-1312.
|
| [10] |
CHEN Changtao, ZHENG Hanxiao, TANG Shengwei,et al.Study on controlling the dissolution of calcium dihydrogen phosphate using ternary phase diagrams to improve the production process of phosphoric acid[J].Industrial & Engineering Chemistry Research,2022,61(43):15970-15979.
|
| [11] |
纪罗军,赵红林.从循环经济角度看工业副产石膏的资源化利用[J].硫酸工业,2021(9):1-8.
|
|
JI Luojun, ZHAO Honglin.Resource utilization of industrial by-product gypsum from the perspective of circular economy[J].Sulphuric Acid Industry,2021(9):1-8.
|
| [12] |
邓华,侯硕旻,李中军,等.磷石膏综合利用现状及展望[J].无机盐工业,2024,56(1):1-8,22.
|
|
DENG Hua, HOU Shuomin, LI Zhongjun,et al.Current situation and prospect of comprehensive utilization of phosphogypsum[J].Inorganic Chemicals Industry,2024,56(1):1-8,22.
|
| [13] |
石鑫,杨绍利,马兰.“三石膏” 综合利用现状及其新工艺[J].现代化工,2020,40(9):8-13,19.
|
|
SHI Xin, YANG Shaoli, MA Lan.Present situation of comprehensive utilization of “three-gypsum” and new technologies[J].Modern Chemical Industry,2020,40(9):8-13,19.
|
| [14] |
张艳丽,张宝林,李冬光.二水硫酸钙结晶过程及活化疏松剂对其影响的研究[J].化工矿物与加工,2004,33(3):11-13.
|
|
ZHANG Yanli, ZHANG Baolin, LI Dongguang.Study on the crystallization course of dihydrate calcium sulphate and the effect of APA on its crystallization[J].Industrial Minerals and Processing,2004,33(3):11-13.
|
| [15] |
HUO Xuejian, GUO Lanfeng, LIU Renlong,et al.Role of additives:Modified hemihydrate phosphogypsum morphology and enhanced filtration performance of wet-process phosphoric acid[J].ACS Omega,2023,8(49):47295-47303.
|
| [16] |
TANG Shuai, JI Yuanhui, GE Kai.Crystallization kinetics and mechanisms of calcium sulfate dihydrate:Experimental investigation and theoretical analysis[J].Industrial & Engineering Chemistry Research,2020,59(50):21676-21684.
|
| [17] |
GAYEVSKII V R, KOCHMARSKII V Z, GAYEVSKA S G.Nucleation and crystal growth of calcium sulfate dihydrate from aqueous solutions:Speciation of solution components,kinetics of growth,and interfacial tension[J].Journal of Crystal Growth,2020,548:125844.
|
| [18] |
SABNIS S S, BANAKAR V V, GOGATE P R,et al.Reactive crystallization of CaCl2 and Na2SO4 in the presence of acoustic cavitation[J].Chemical Engineering and Processing - Process Intensification,2022,170:108702.
|
| [19] |
崔益顺,付凌杰,李国勇,等.响应曲面法优化工业高钙废水制硫酸钙晶须的工艺[J].环境工程学报,2015,9(10):4828-4832.
|
|
CUI Yishun, FU Lingjie, LI Guoyong,et al.Optimization on preparation of calcium sulphate whiskers from high calcium wastewater by using response surface method[J].Chinese Journal of Environmental Engineering,2015,9(10):4828-4832.
|
 ), ZHOU Rong2, LÜ Li1(
), ZHOU Rong2, LÜ Li1( ), YANG Jie1, TANG Shengwei1, ZHANG Tao1
), YANG Jie1, TANG Shengwei1, ZHANG Tao1