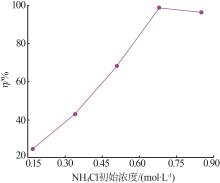
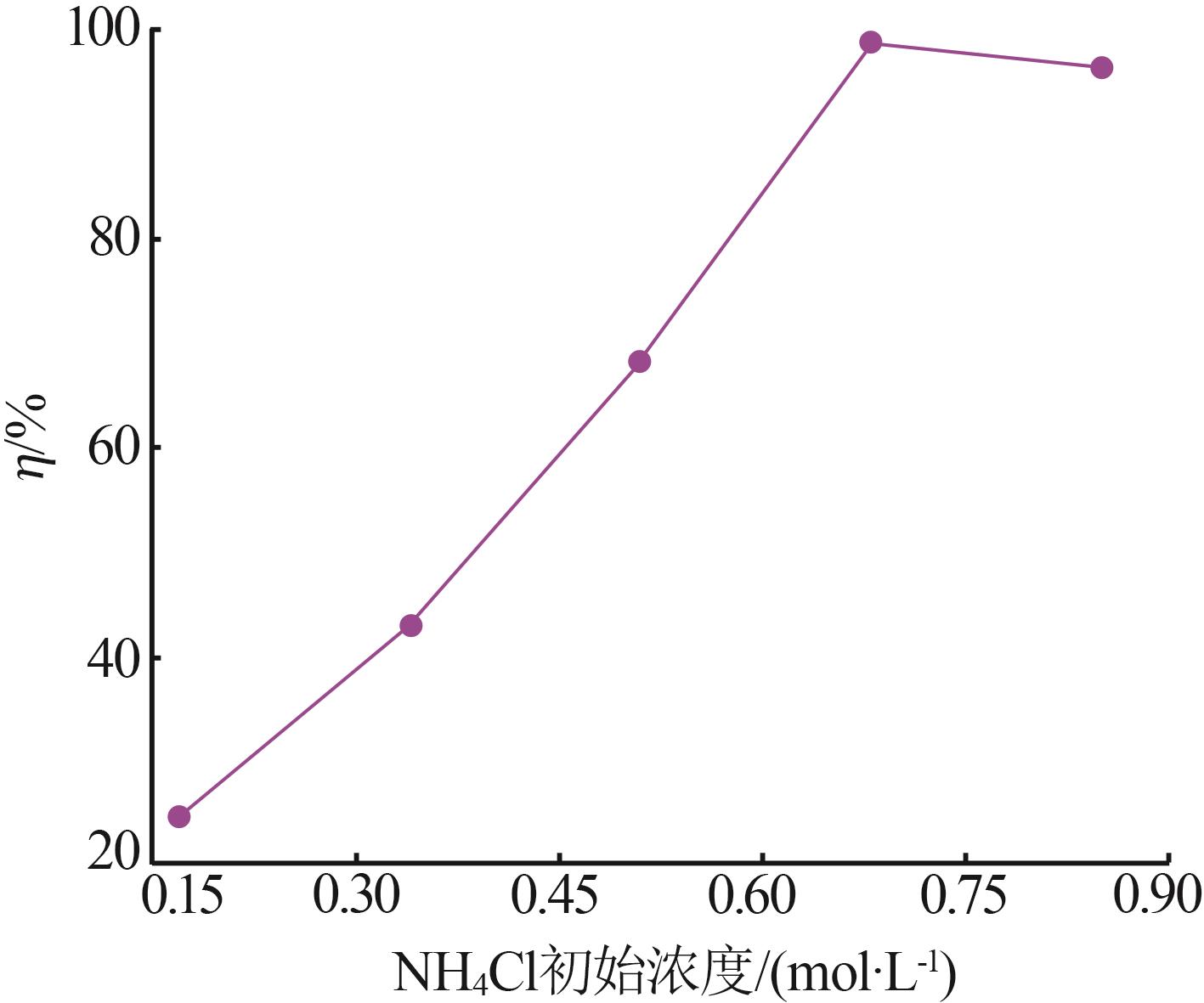
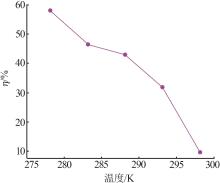
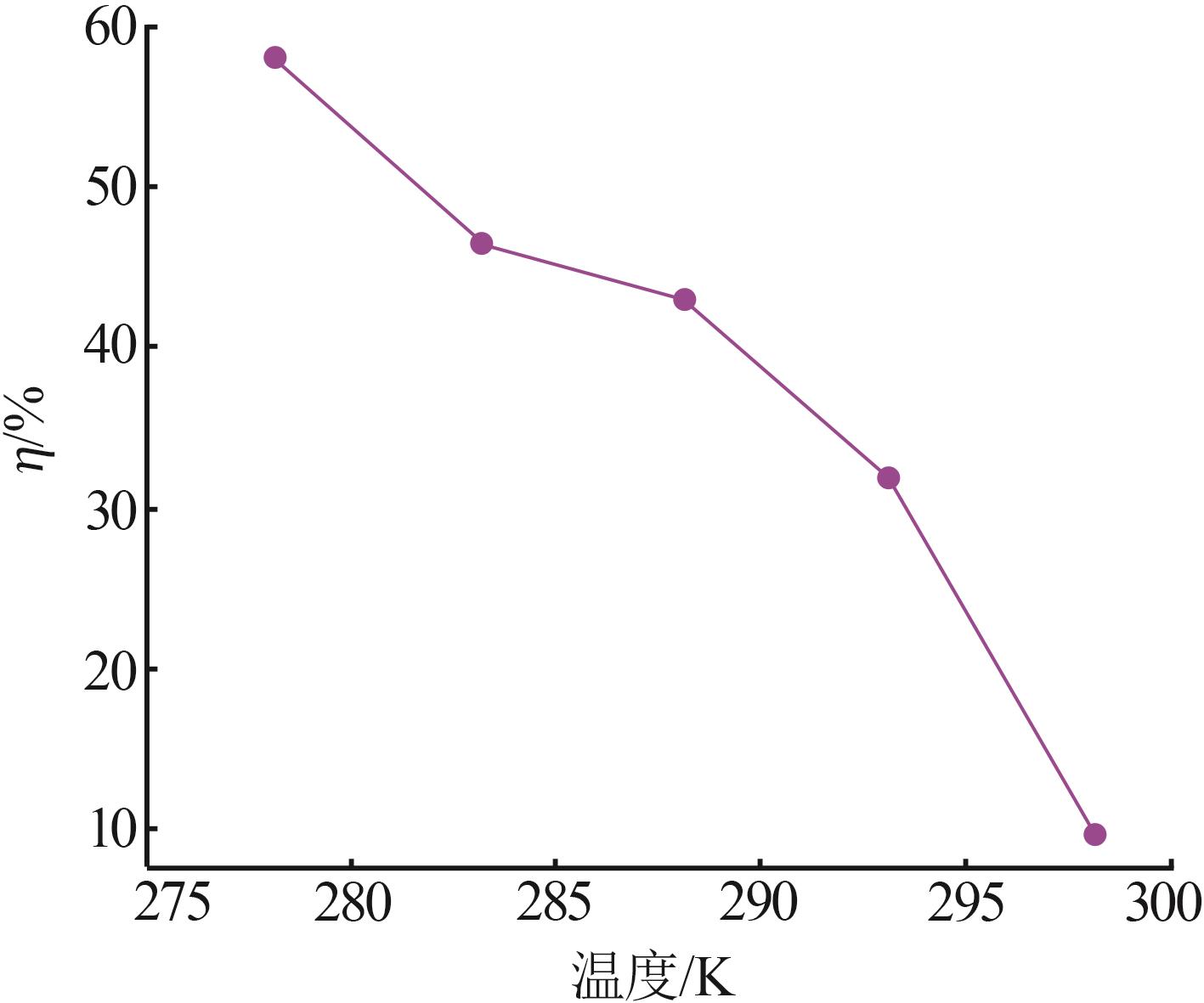
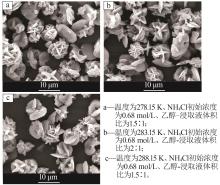
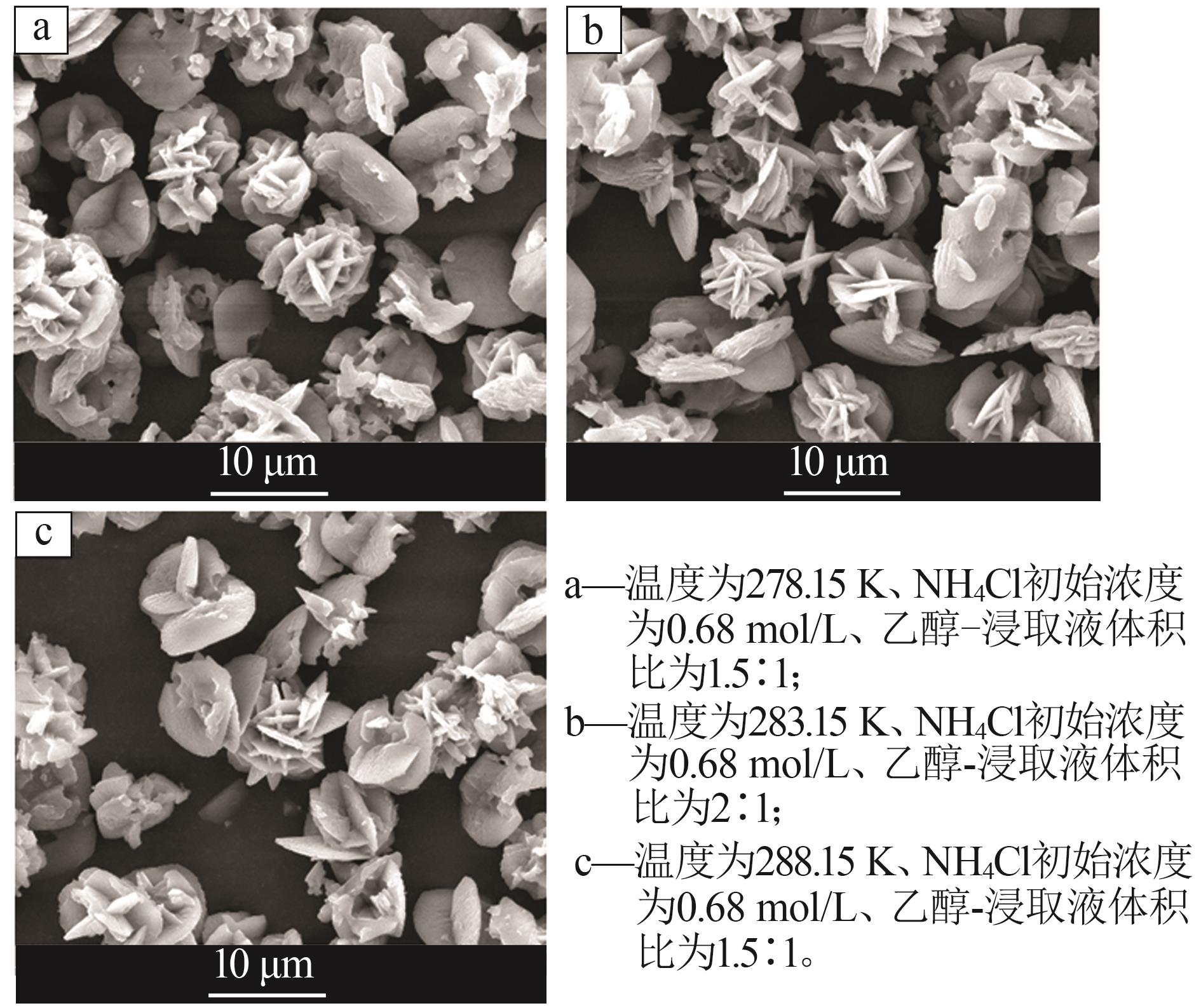
| 1 |
BARAN R, MILLOT Y, AVERSENG F,et al.Vanadium incorporation from aqueous NH4VO3 solution into siliceous Beta zeolite determined by NMR with formation of V-single site zeolite catalysts for application in SCR of NO[J].Applied Catalysis A:General,2020,606:117830.
|
| 2 |
MAHTAB M, DAVOOD H, MEHDI B,et al.ZrCl4 or NH4VO3 as a versatile catalyst for the capable synthesis of xanthenediones and their corresponding theoretical studies[J].Inorganic Chemistry Communications,2022,141:109582.
|
| 3 |
官清,王远望,彭荣华,等.用偏钒酸铵制备偏钒酸钠工艺研究[J].无机盐工业,2016,48(9):57-60.
|
|
GUAN Qing, WANG Yuanwang, PENG Ronghua,et al.Study on preparation technology of sodium metavanadate with ammonium metavanadate[J].Inorganic Chemicals Industry,2016,48(9):57- 60.
|
| 4 |
DENG Rongrui, XIAO Hao, XIE Zhaoming,et al.A novel method for extracting vanadium by low temperature sodium roasting from converter vanadium slag[J].Chinese Journal of Chemical Engineering,2020,28(8):2208-2213.
|
| 5 |
LI Hongyi, WANG Chengjie, LIN Minmin,et al.Green one-step roasting method for efficient extraction of vanadium and chromium from vanadium-chromium slag[J].Powder Technology,2020,360:503-508.
|
| 6 |
杜小旺,仲剑初,王孝天.高硅水镁石钠化焙烧法除硅工艺研究[J].无机盐工业,2020,52(10):92-95.
|
|
DU Xiaowang, ZHONG Jianchu, WANG Xiaotian.Study on removal of silicon from high-silicon brucite by sodium roasting method[J].Inorganic Chemicals Industry,2020,52(10):92-95.
|
| 7 |
MENG Yuqi, WANG Xuewen, YANG Minge,et al.Recovery of Cr from vanadium-containing chromate solution with copper salt precipitation after V separation[J].Hydrometallurgy,2019,188:157- 160.
|
| 8 |
LI Hailong, FENG Yali, WANG Hongjun,et al.Separation of V(Ⅴ) and Mo(Ⅵ) in roasting-water leaching solution of spent hydrodesulfurization catalyst by co-extraction using P507-N235 extractant[J].Separation and Purification Technology,2020,248:117135.
|
| 9 |
ZHANG Yutao, ZHAO Ruzhen, ZHANG Xiaohuan,et al.A novel technology for producing high-purity V2O5 from hazardous vanadium-containing solutions using precipitation and solvent extraction[J].Process Safety and Environmental Protection,2023,173:567-578.
|
| 10 |
YING Ziwen, HUO Manxing, WU Guixuan,et al.Recovery of vanadium and chromium from leaching solution of sodium roasting vanadium slag by stepwise separation using amide and EHEHPA[J].Separation and Purification Technology,2021,269:118741.
|
| 11 |
张焕焕,杜光文,吴然昊,等.Aliquat 336萃取提钒性能研究[J].应用化工,2022,51(10):2855-2858.
|
|
ZHANG Huanhuan, DU Guangwen, WU Ranhao,et al.Study on extraction properties of vanadium for Aliquat 336[J].Applied Chemical Industry,2022,51(10):2855-2858.
|
| 12 |
WOLOWICZ A, HUBICKI Z.Removal of vanadium by ion exchange resins from model and real solutions from spent V2O5 catalyst[J].Hydrometallurgy,2022,211:105871.
|
| 13 |
LI Hongyi, LI Cui, ZHANG Meng,et al.Removal of V(Ⅴ) from aqueous Cr(Ⅵ)-bearing solution using anion exchange resin:Equilibrium and kinetics in batch studies[J].Hydrometallurgy,2016,165:381-389.
|
| 14 |
WANG Xuewen, WANG Mingyu, SHI Lihua,et al.Recovery of vanadium during ammonium molybdate production using ion exchange[J].Hydrometallurgy,2010,104(2):317-321.
|
| 15 |
郭雪梅,王少娜,杜浩,等.碳酸氢铵溶液中偏钒酸铵的冷却结晶[J].化工进展,2018,37(3):853-860.
|
|
GUO Xuemei, WANG Shaona, DU Hao,et al.Cooling crystallization of ammonium metavanadate from ammonium bicarbonate solution[J].Chemical Industry and Engineering Progress,2018,37(3):853-860.
|
| 16 |
张峰榛,张焕焕,程卓,等.一种高纯偏钒酸铵晶体及制备方法:中国,114162865A[P].2022-03-11.
|
| 17 |
李锐,姜永华,张燕玲,等.基于响应曲面法优化硫酸铵结晶[J].硅酸盐学报,2022,50(3):782-790.
|
|
LI Rui, JIANG Yonghua, ZHANG Yanling,et al.Optimisation of ammonium sulphate crystallization based on response surface methodology[J].Journal of the Chinese Ceramic Society,2022,50(3):782-790.
|
 ), ZHANG Huanhuan, CHENG Zhuo, TANG Xiuhua, ZHANG Fengzhen(
), ZHANG Huanhuan, CHENG Zhuo, TANG Xiuhua, ZHANG Fengzhen( ), YE Yuling
), YE Yuling