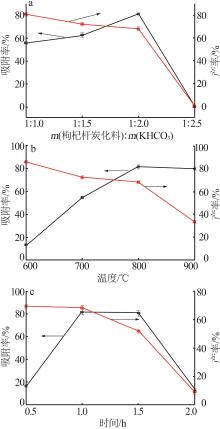
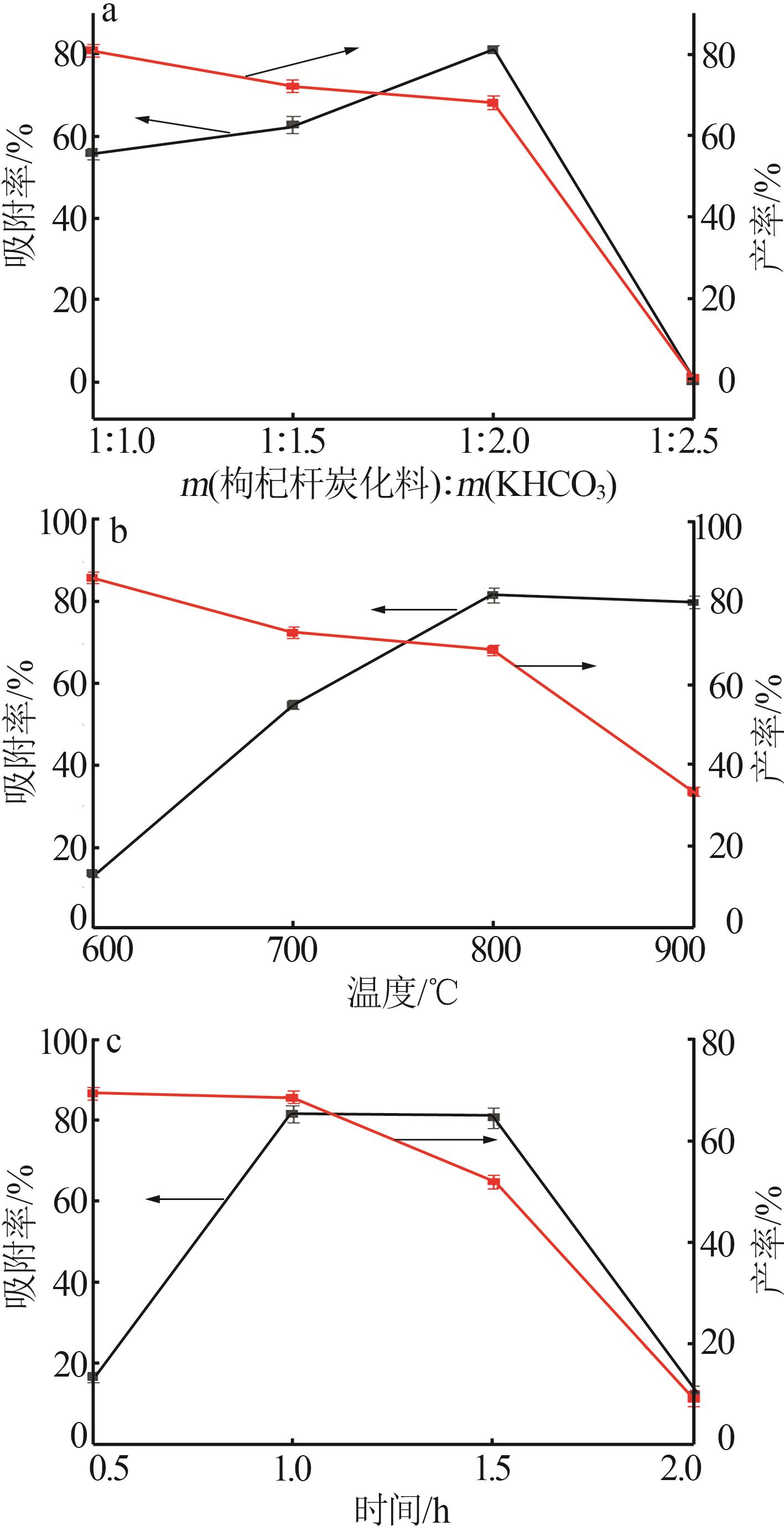
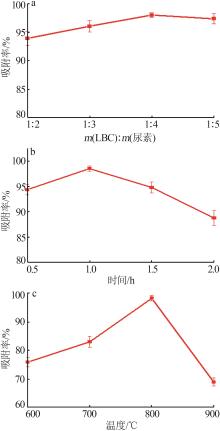
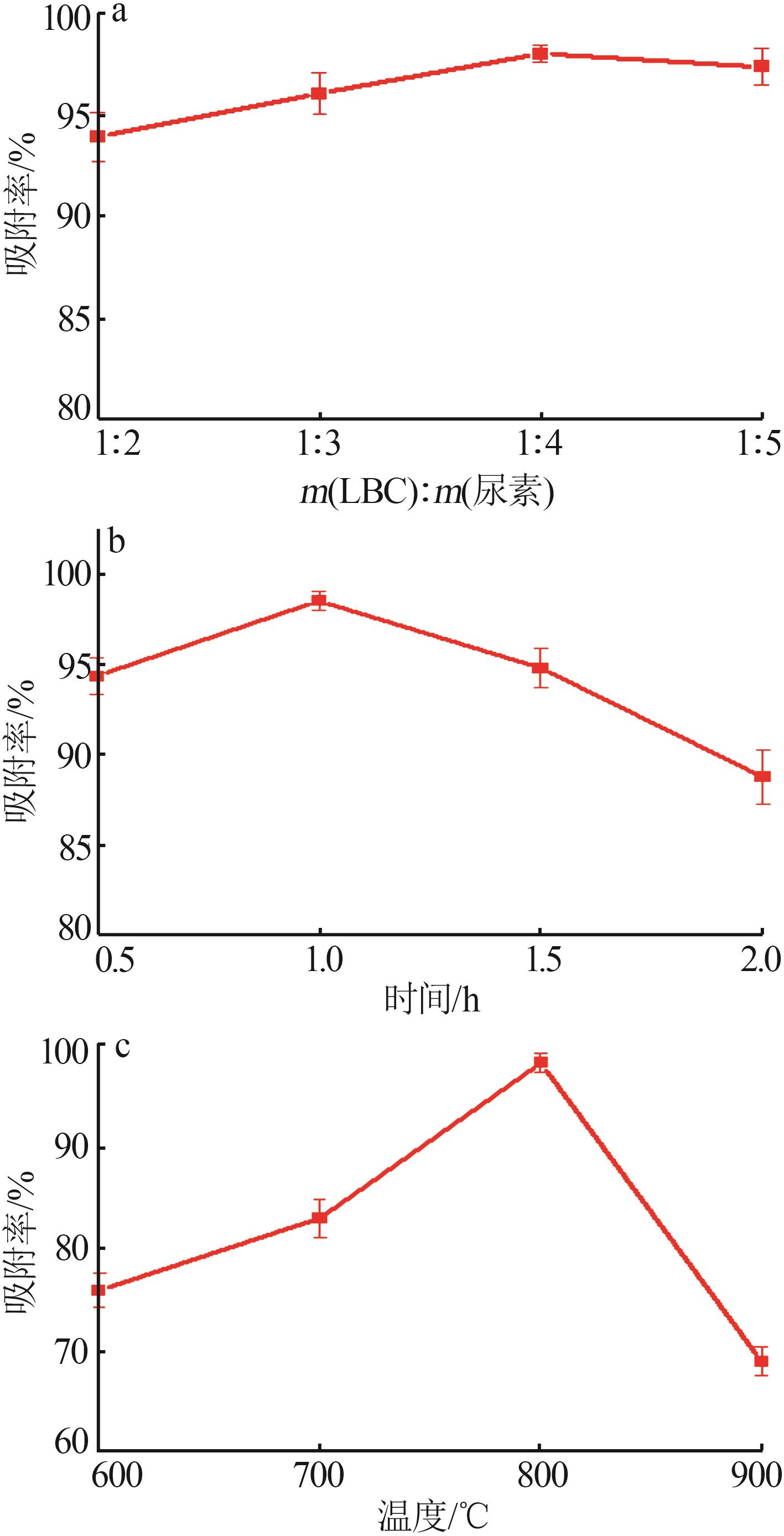
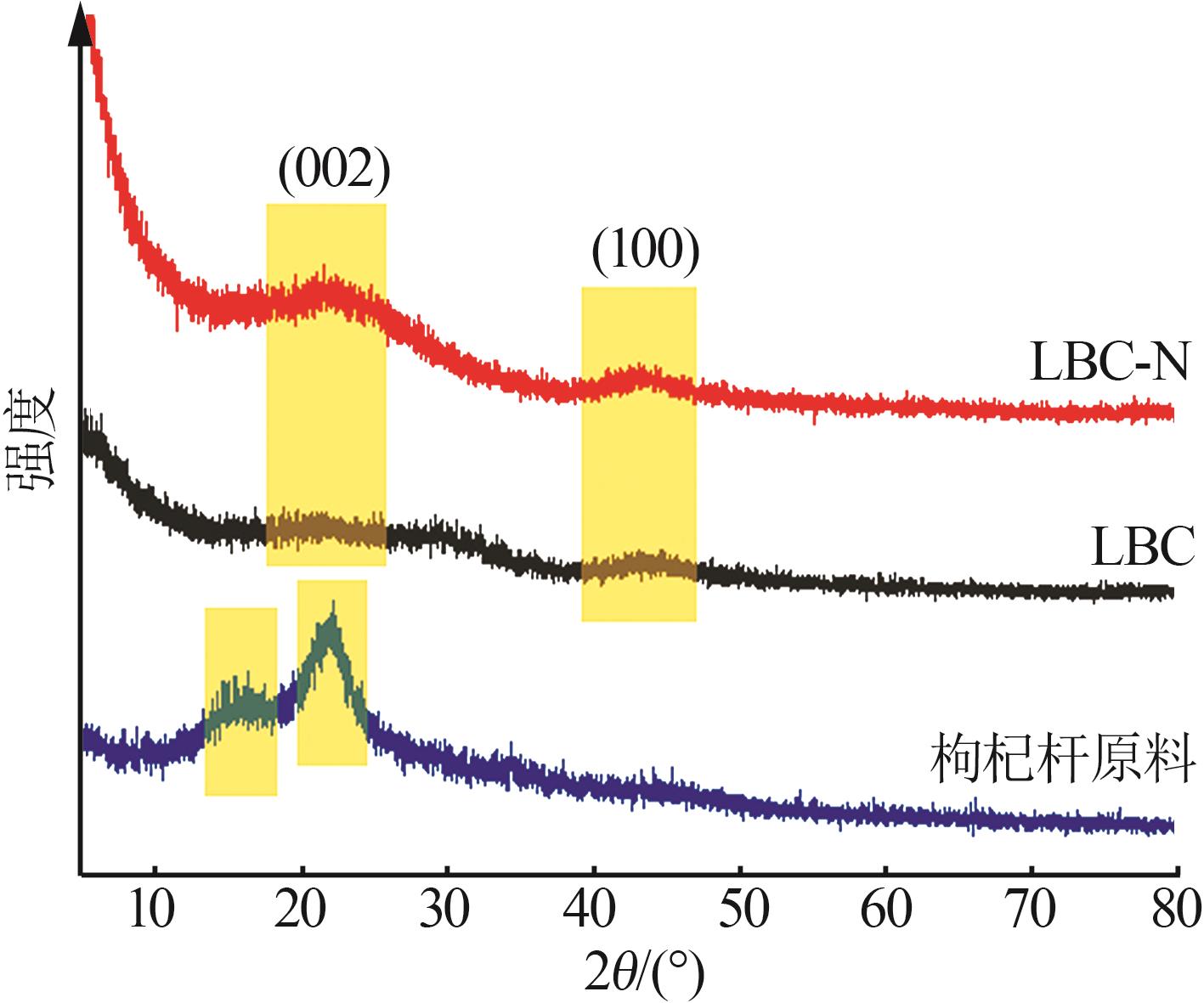
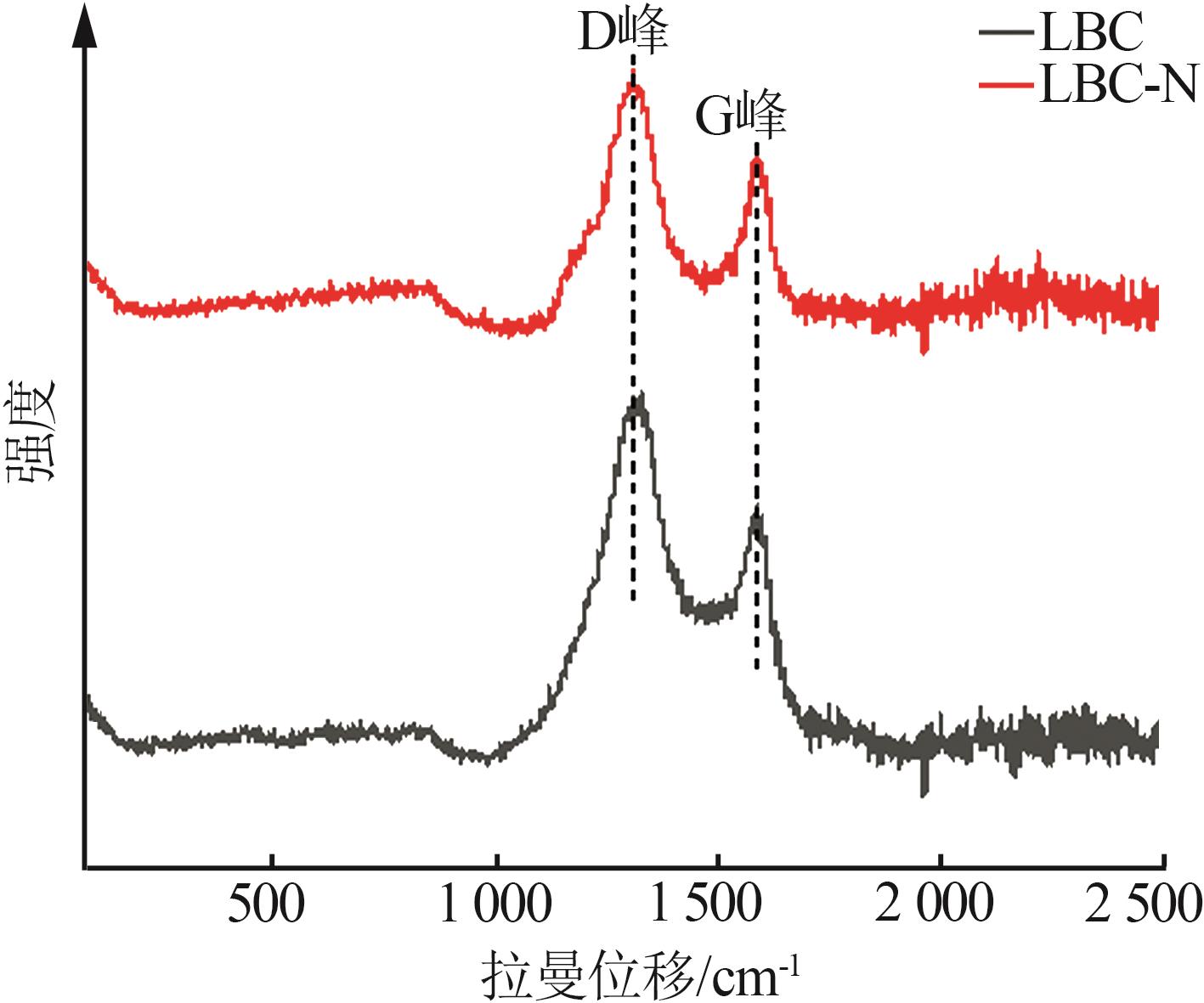
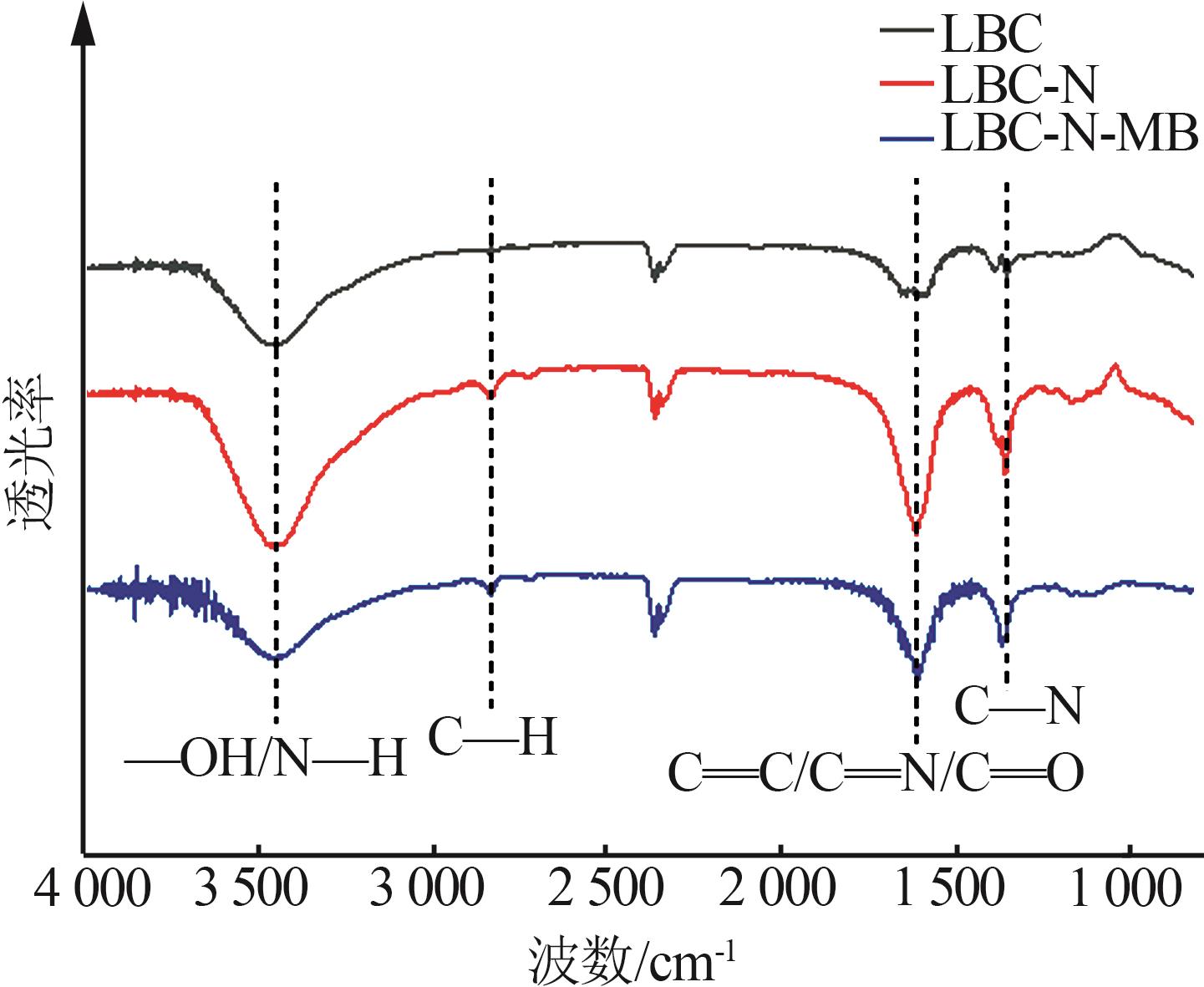
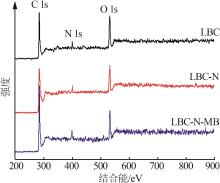
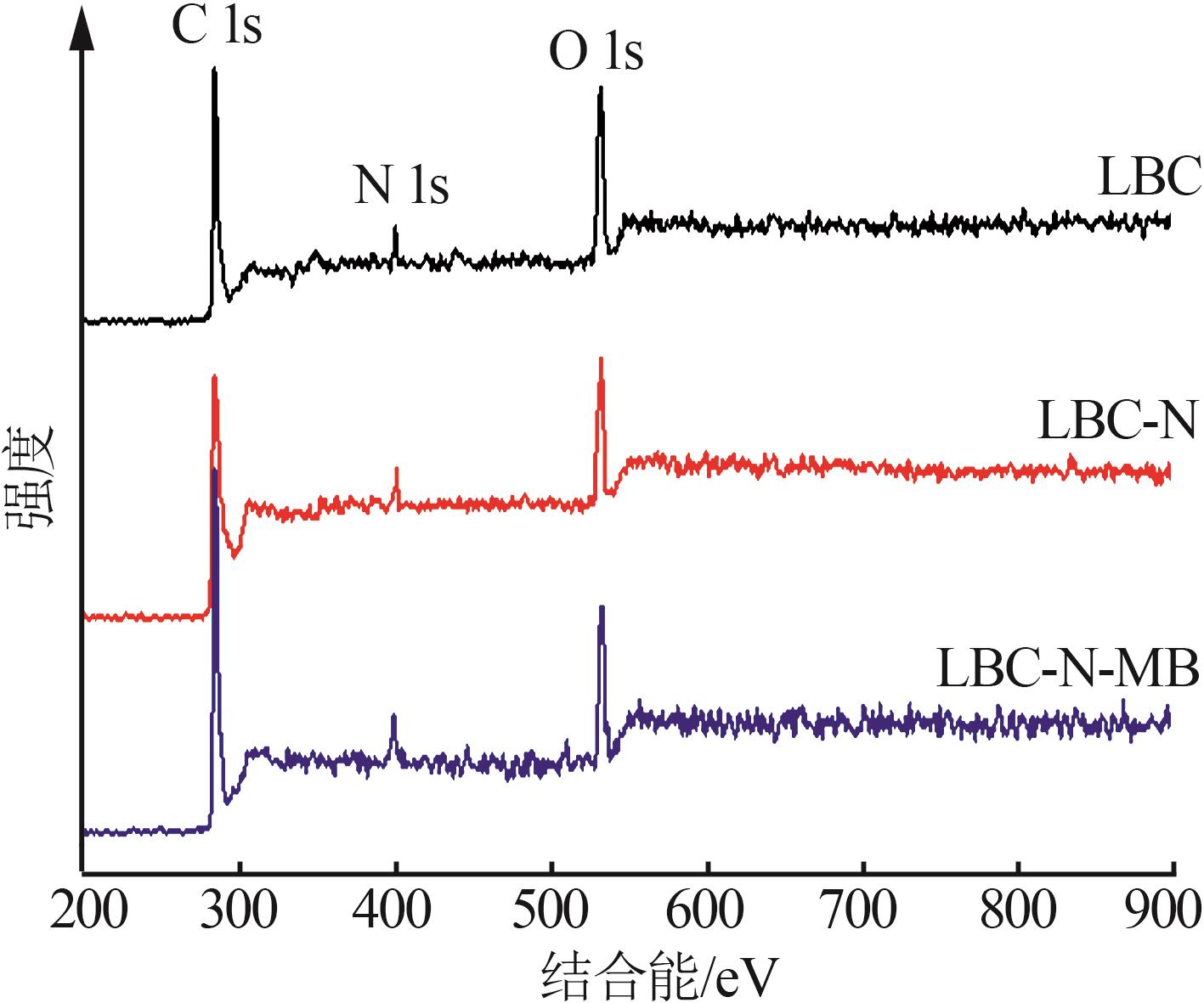
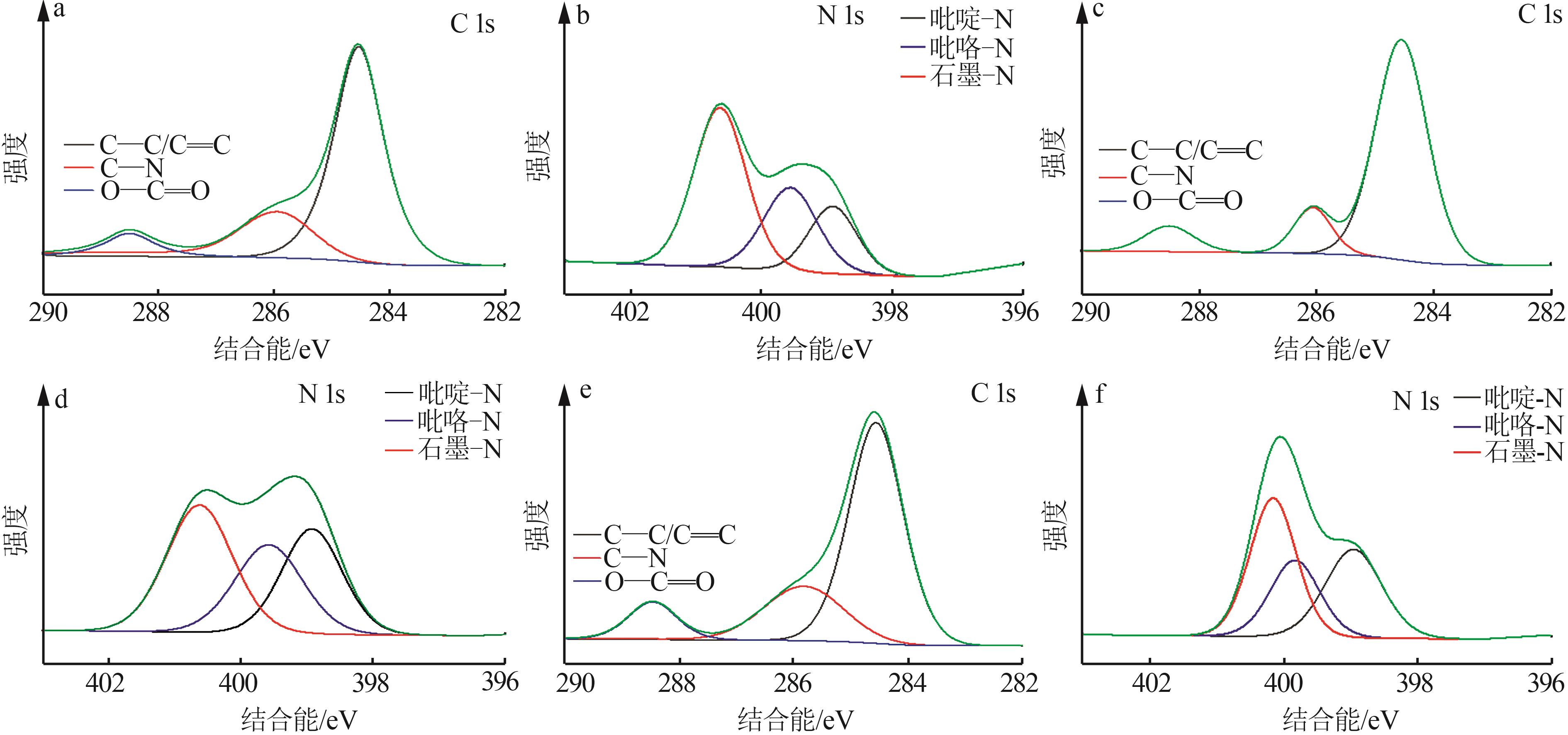
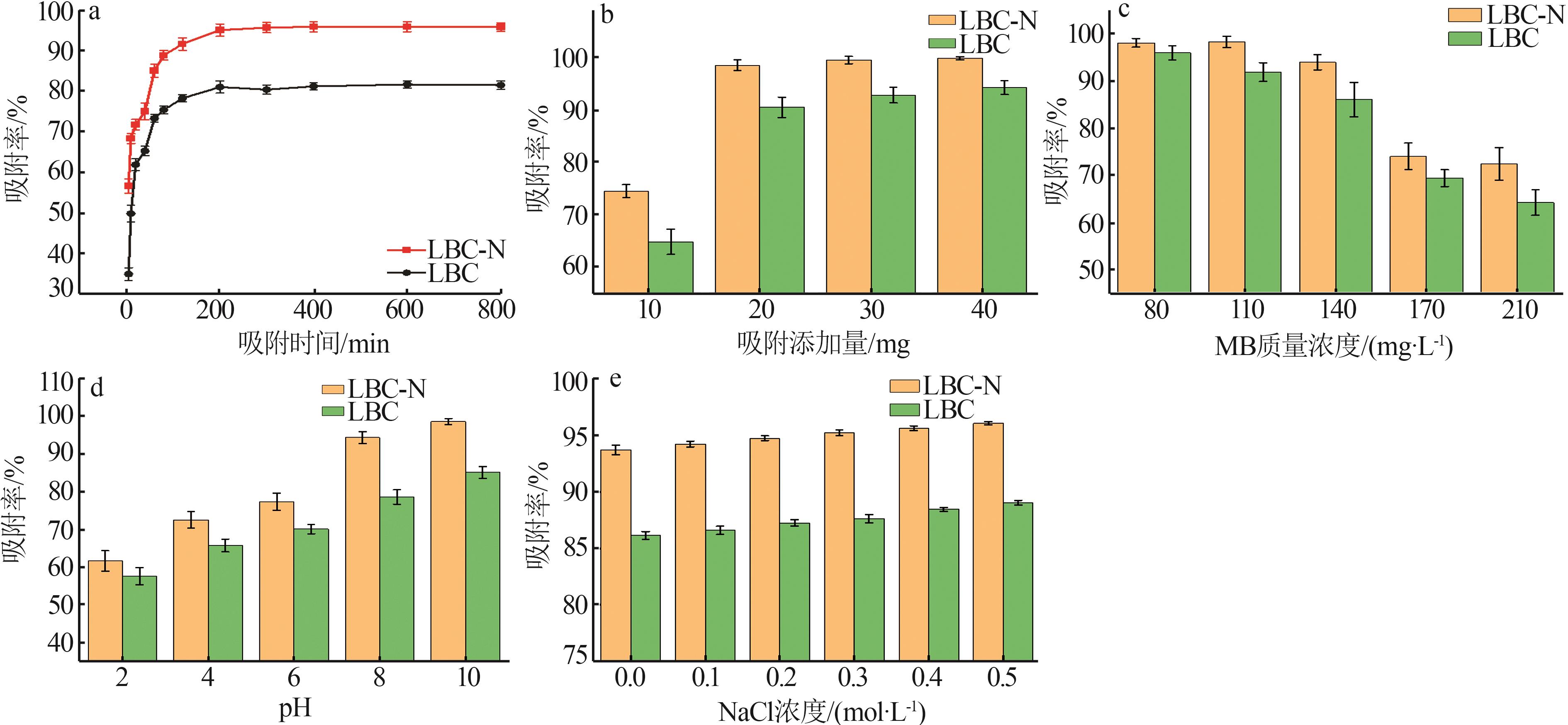
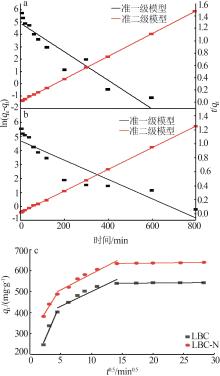
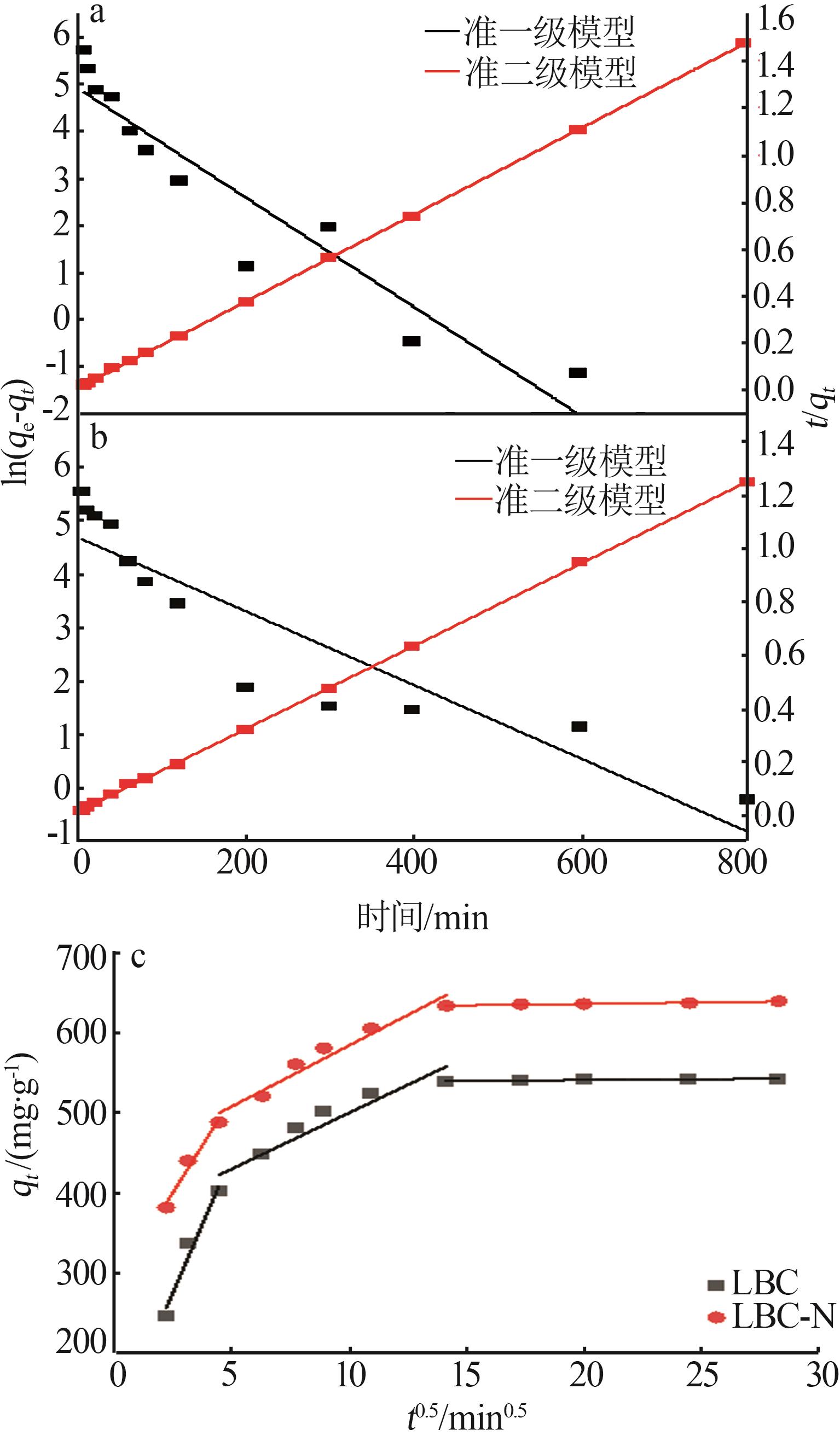
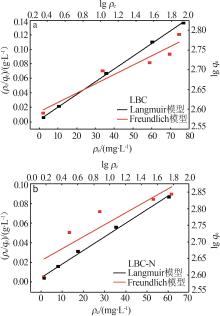
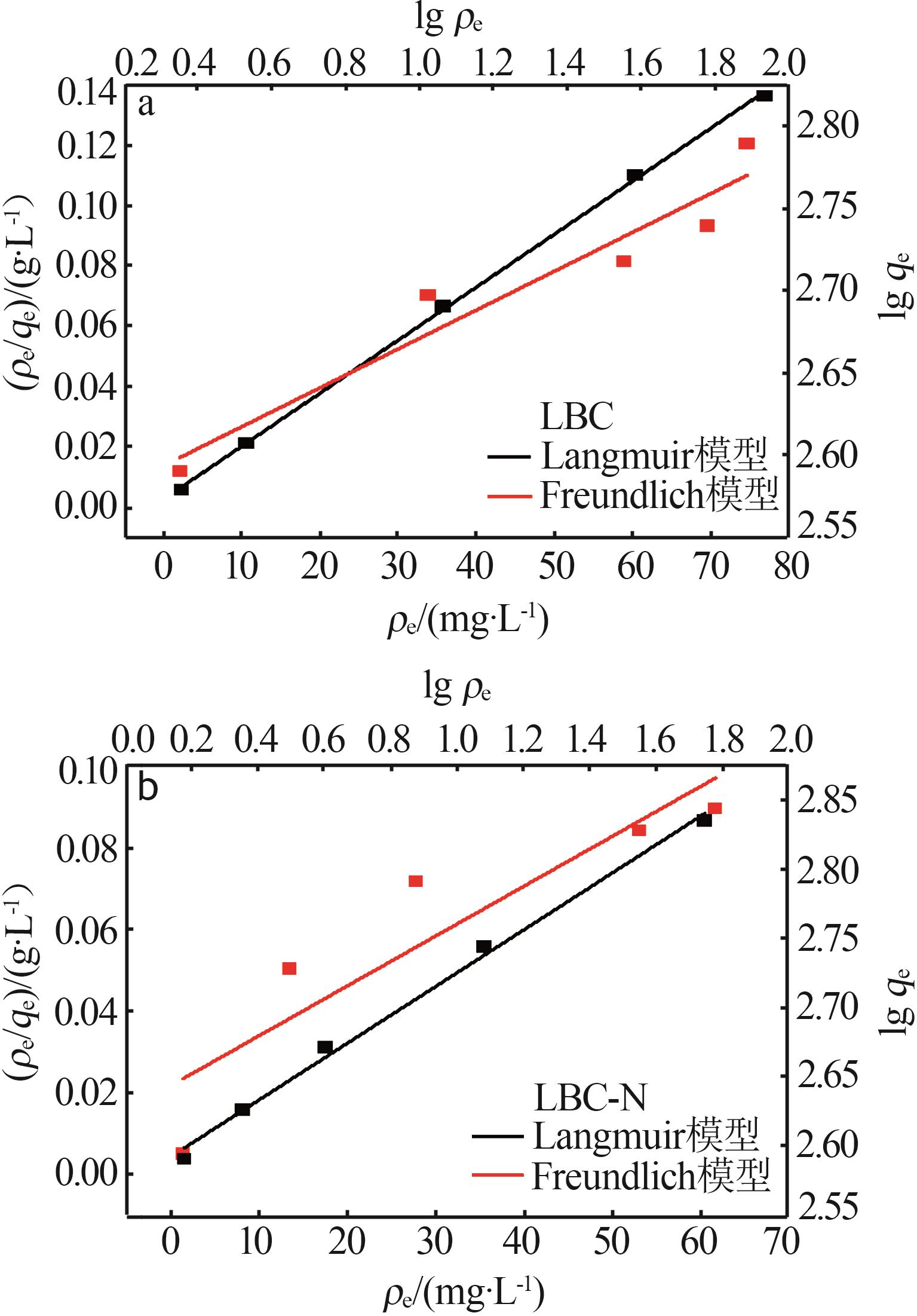
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (9): 117-127.doi: 10.19964/j.issn.1006-4990.2024-0001
• Environment·Health·Safety • Previous Articles Next Articles
Study on preparation of nitrogen-doped biochar and its adsorption properties for methylene blue
WANG Ping1( ), XU Rongsheng1,2(
), XU Rongsheng1,2( ), SUN Dong1, SHI Xiaohong1, XU Wei1, LI Mei1
), SUN Dong1, SHI Xiaohong1, XU Wei1, LI Mei1
- 1.School of Chemistry and Chemical Engineering,North Minzu University,Yinchuan 750021,China
2.Key Laboratory for Chemical Engineering and Technology,State Ethnic Affairs Commission,North Minzu University,Yinchuan 750021,China
-
Received:2024-01-02Online:2024-09-10Published:2024-04-11 -
Contact:XU Rongsheng E-mail:w0917pp@163.com;xurongsheng6463@163.com
CLC Number:
Cite this article
WANG Ping, XU Rongsheng, SUN Dong, SHI Xiaohong, XU Wei, LI Mei. Study on preparation of nitrogen-doped biochar and its adsorption properties for methylene blue[J]. Inorganic Chemicals Industry, 2024, 56(9): 117-127.
share this article
| 1 | LIAN Fei, CUI Guannan, LIU Zhongqi,et al.One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity[J].Journal of Environmental Management,2016,176:61-68. |
| 2 | ZHENG Wanlan, CHEN Shuang, LIU Huie,et al.Study of the modification mechanism of heavy metal ions adsorbed by biomass-activated carbon doped with a solid nitrogen source[J].RSC Advances,2019,9(64):37440-37449. |
| 3 | 梁波,王清华,朱凯,等.咖啡渣活性炭对亚甲基蓝吸附特性研究[J].工业用水与废水,2023,54(3):16-20. |
| LIANG Bo, WANG Qinghua, ZHU Kai,et al.Adsorption characteristics of coffee grounds activated carbon for methylene blue[J].Industrial Water & Wastewater,2023,54(3):16-20. | |
| 4 | 陈关霄,吕燕根.花生壳基活性炭的制备及吸附孔雀石绿性能分析[J].福建技术师范学院学报,2021,39(5):457-463. |
| CHEN Guanxiao, LV Yangen.A study on the preparation of peanut-shell-based activated carbon and its adsorption performance ofMG[J].Journal of Fujian Polytechnic Normal University,2021,39(5):457-463. | |
| 5 | BAI Xiaoli, QUAN Bingyan, KANG Chaoyan,et al.Activated carbon from tea residue as efficient absorbents for environmental pollutant removal from wastewater[J].Biomass Conversion and Biorefinery,2023,13(15):13433-13442. |
| 6 | YE Shujing, ZENG Guangming, TAN Xiaofei,et al.Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer[J].Applied Catalysis B:Environmental,2020,269:118850. |
| 7 | ZHANG Xiong, ZHANG Shihong, YANG Haiping,et al.Generalized two-dimensional correlation infrared spectroscopy to reveal mechanisms of CO2 capture in nitrogen enriched biochar[J].Proceedings of the Combustion Institute,2017,36(3):3933-3940. |
| 8 | 郝宏蕾,孟繁雨,李若钰,等.生物质基氮掺杂多孔炭材料的制备及对水中亚甲基蓝的吸附性能[J].高等学校化学学报,2022,43(6):226-236. |
| HAO Honglei, MENG Fanyu, LI Ruoyu,et al.Biomass derived nitrogen doped porous carbon materials as adsorbents for removal of methylene blue in water[J].Chemical Journal of Chinese Universities,2022,43(6):226-236. | |
| 9 | LI Yunchao, XING Bo, WANG Xiaoliu,et al.Nitrogen-doped hierarchical porous biochar derived from corn stalks for phenol-enhanced adsorption[J].Energy & Fuels,2019,33(12):12459-12468. |
| 10 | 代祥,罗婴棋,张文俊,等.氮掺杂生物炭去除水中六价铬的效果及机理[J].环境科学学报,2023,43(11):84-93. |
| DAI Xiang, LUO Yingqi, ZHANG Wenjun,et al.Removal of hexavalent chromium from water using nitrogen-doped biochar:Performance and mechanism[J].Acta Scientiae Circumstantiae,2023,43(11):84-93. | |
| 11 | 冯倩,徐荣声,李梅,等.向日葵制活性炭对亚甲基蓝的吸附研究[J].无机盐工业,2021,53(12):122-128. |
| FENG Qian, XU Rongsheng, LI Mei,et al.Research on adsorption of methylene blue on activated carbon prepared from sunflower[J].Inorganic Chemicals Industry,2021,53(12):122-128. | |
| 12 | YING Zhiwei, CHEN Xinwei, LI He,et al.Efficient adsorption of methylene blue by porous biochar derived from soybean dreg using a one-pot synthesis method[J].Molecules,2021,26(3):661. |
| 13 | 黄毓颖,李坤权,姚文,等.碳酸氢钾活化制备水稻秸秆炭孔结构的影响因素及吸附性能[J].环境化学,2018,37(3):569-575. |
| HUANG Yuying, LI Kunquan, YAO Wen,et al.Investigation on the influencing factors of pore structure on rice straw activated carbon with KHCO3 and its adsorption ability[J].Environmental Chemistry,2018,37(3):569-575. | |
| 14 | 余谟鑫,张书海,朱博文,等.N掺杂生物炭的制备及其对Co2+的吸附性能[J].材料研究学报,2023,37(4):291-300. |
| YU Moxin, ZHANG Shuhai, ZHU Bowen,et al.Preparation of nitrogen-doped biochar and its adsorption capacity for Co2+ [J].Chinese Journal of Materials Research,2023,37(4):291-300. | |
| 15 | OH W D, LISAK G, WEBSTER R D,et al.Insights into the thermolytic transformation of lignocellulosic biomass waste to redox-active carbocatalyst:Durability of surface active sites[J].Applied Catalysis B:Environmental,2018,233:120-129. |
| 16 | CHENG Youliang, ZHANG Qingling, FANG Changqing,et al.Synthesis of N-doped porous carbon materials derived from waste cellulose acetate fiber via urea activation and its potential application in supercapacitors[J].Journal of the Electrochemical Society,2019,166(6):A1231-A1238. |
| 17 | LI Jing, LV Fanxun, YANG R,et al.N-doped biochar from lignocellulosic biomass for preparation of adsorbent:Characterization,kinetics and application[J].Polymers,2022,14(18):3889. |
| 18 | 徐荣声,孟泽,冯倩,等.氯化锌-水蒸气协同活化玉米芯制活性炭的研究[J].无机盐工业,2023,55(12):119-127. |
| XU Rongsheng, MENG Ze, FENG Qian,et al.Study on preparation of activated carbon from corn cob by zinc chloride and water vapor[J].Inorganic Chemicals Industry,2023,55(12):119-127. | |
| 19 | MI Bingbing, WANG Jingxin, XIANG Hongzhong,et al.Nitrogen self-doped activated carbons derived from bamboo shoots as adsorbent for methylene blue adsorption[J].Molecules,2019,24(16):3012. |
| [1] | BAI Xingxing, LI Hanfei, TANG Yong, ZHANG Jun, ZHU Guangkai, LI Lishuo, TONG Zhangfa. Study on preparation of cellulose based hydrogel doped with nano-calcium carbonate and its adsorption properties of copper ions [J]. Inorganic Chemicals Industry, 2025, 57(2): 83-91. |
| [2] | LUO Chengling, FAN Xiaofan. Research progress of microstructure-regulated catalysts for urea oxidation reactions [J]. Inorganic Chemicals Industry, 2025, 57(2): 26-35. |
| [3] | ZHANG Feigang, LIU Zhongli. Study on application of CuO/g-C3N4 composites in organic dye degradation and supercapacitors [J]. Inorganic Chemicals Industry, 2025, 57(1): 129-136. |
| [4] | SONG Zhaoxia, LIU Yongkang, GUO Yaokun, WANG Tengfei. Study on adsorption performance of wheat straw biochar on malachite green [J]. Inorganic Chemicals Industry, 2024, 56(9): 128-135. |
| [5] | XIONG Chengrong, CHEN Yan, TANG Miao, ZHANG Fengze, ZHOU Tianxiang, OUYANG Ruifeng, DONG Gang, SHI Wei, ZENG Tao, CHEN Yunxia, SU Xiaoli. Research progress on preparation of 2D vermiculite nanosheets and their environmental adsorption of pollutants [J]. Inorganic Chemicals Industry, 2024, 56(8): 19-26. |
| [6] | PANG Hongchang, LIU Shuai, TIAN Peng, NING Guiling. Study on application of modified halloysite nanotubes in polyurea⁃based fireproof coatings [J]. Inorganic Chemicals Industry, 2024, 56(8): 27-32. |
| [7] | ZHANG Bangcheng, WANG Li. Preparation and adsorption properties of waste polyester⁃based activated carbon activated by ZnCl2 [J]. Inorganic Chemicals Industry, 2024, 56(7): 126-134. |
| [8] | ZHANG Lijin, LÜ Qing, CHEN Xiaolang, LI Qingxin, SHI Hongyu, QIN Jun. Preparation of Ca-based LDO composite material and its adsorption performance for phosphate [J]. Inorganic Chemicals Industry, 2024, 56(7): 37-45. |
| [9] | ZHAO Chuang, ZHANG Boyu, LI Ben, JIN Fengying, LI Bin, SUN Zhenhai, GUO Chunlei. Study on adsorption and separation technology of polycyclic aromatic hydrocarbons by adsorbent [J]. Inorganic Chemicals Industry, 2024, 56(7): 61-68. |
| [10] | LIU Kailong, ZHU Kongyi, GUO Chunlei, MA Xiaobiao, WANG Yujian, SHENG Qiang, LI Xiang, WANG Yinbin, JIN Fengying. Effects of process condition on performance of diesel aromatic to light aromatic [J]. Inorganic Chemicals Industry, 2024, 56(6): 139-146. |
| [11] | ZHU Zenghu, WANG Min, PENG Zhengjun, JIA Guofeng, LI Yan. Study on adsorption of potassium ions in lithium chloride solution [J]. Inorganic Chemicals Industry, 2024, 56(6): 61-66. |
| [12] | XU Mengyao, ZHANG Xin, HE Kunpeng, HE Jian, JIANG Wei. Preparation of yttrium oxide and zirconium phosphate adsorbents from zirconium-yttrium waste and evaluation of their performance [J]. Inorganic Chemicals Industry, 2024, 56(3): 116-124. |
| [13] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [14] | HUANG Jianan, LU Xiaoyu, WANG Mitang. Effect of Ba-La co-doping on degradation of methylene blue dye by TaON [J]. Inorganic Chemicals Industry, 2024, 56(2): 146-151. |
| [15] | LI Yang, LOU Feijian, SUI Xin, LI Keyan, LIU Fei, GUO Xinwen. Preparation of amine-functionalized fumed SiO2 materials and their performance for CO2 adsorption [J]. Inorganic Chemicals Industry, 2024, 56(2): 38-43. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||