Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (10): 103-110.doi: 10.19964/j.issn.1006-4990.2024-0531
• Environment·Health·Safety • Previous Articles Next Articles
Study on evaporation and concentration characteristics of desulfurization wastewater
CHEN Haijie1( ), MA Xiaoyue1, WEI Xin1, SUN Congcong2, YU Zikai2, YANG Jing2(
), MA Xiaoyue1, WEI Xin1, SUN Congcong2, YU Zikai2, YANG Jing2( ), WANG Yanfei2(
), WANG Yanfei2( )
)
- 1. Datang Environmental Industry Group Co. ,Ltd. ,Beijing 100097,China
2. Tianjin University of Science and Technology,Tianjin 300457,China
-
Received:2024-10-09Online:2025-10-10Published:2025-10-27 -
Contact:YANG Jing, WANG Yanfei E-mail:chenhj@dteg.com.cn;yang_J@tust.edu.cn;wangyanfei@tust.edu.cn
CLC Number:
Cite this article
CHEN Haijie, MA Xiaoyue, WEI Xin, SUN Congcong, YU Zikai, YANG Jing, WANG Yanfei. Study on evaporation and concentration characteristics of desulfurization wastewater[J]. Inorganic Chemicals Industry, 2025, 57(10): 103-110.
share this article
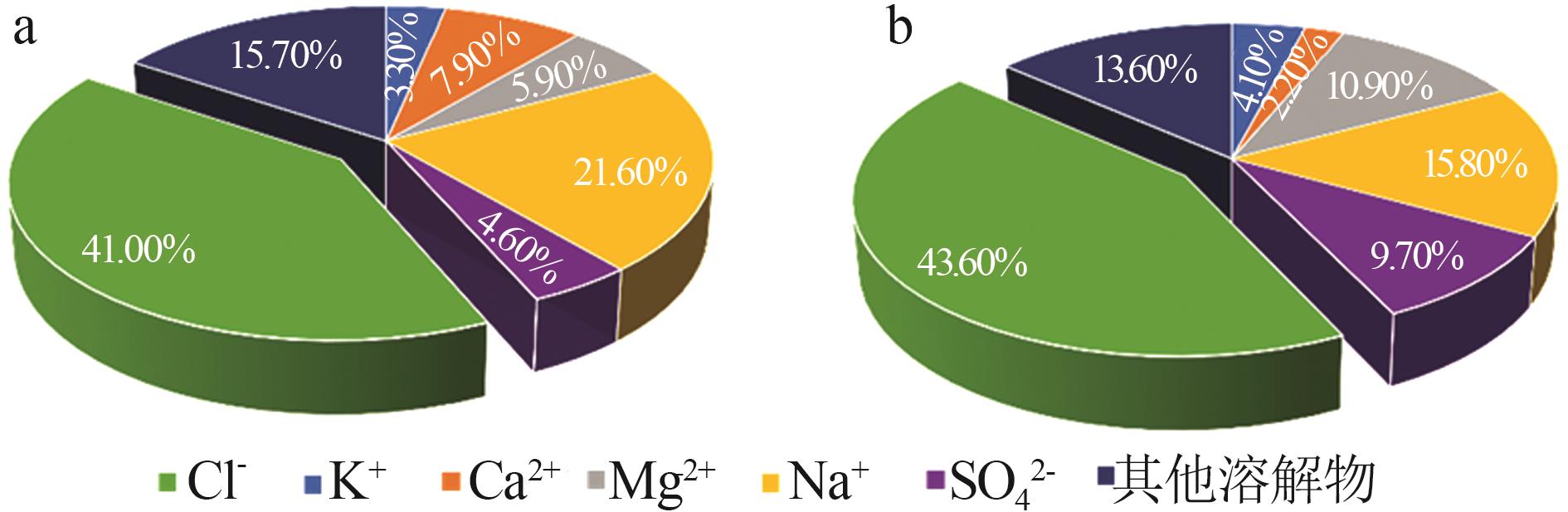
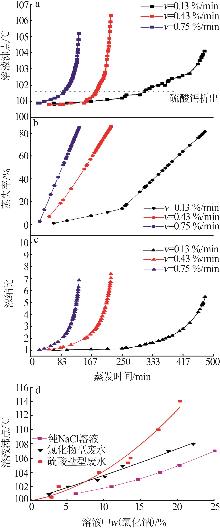
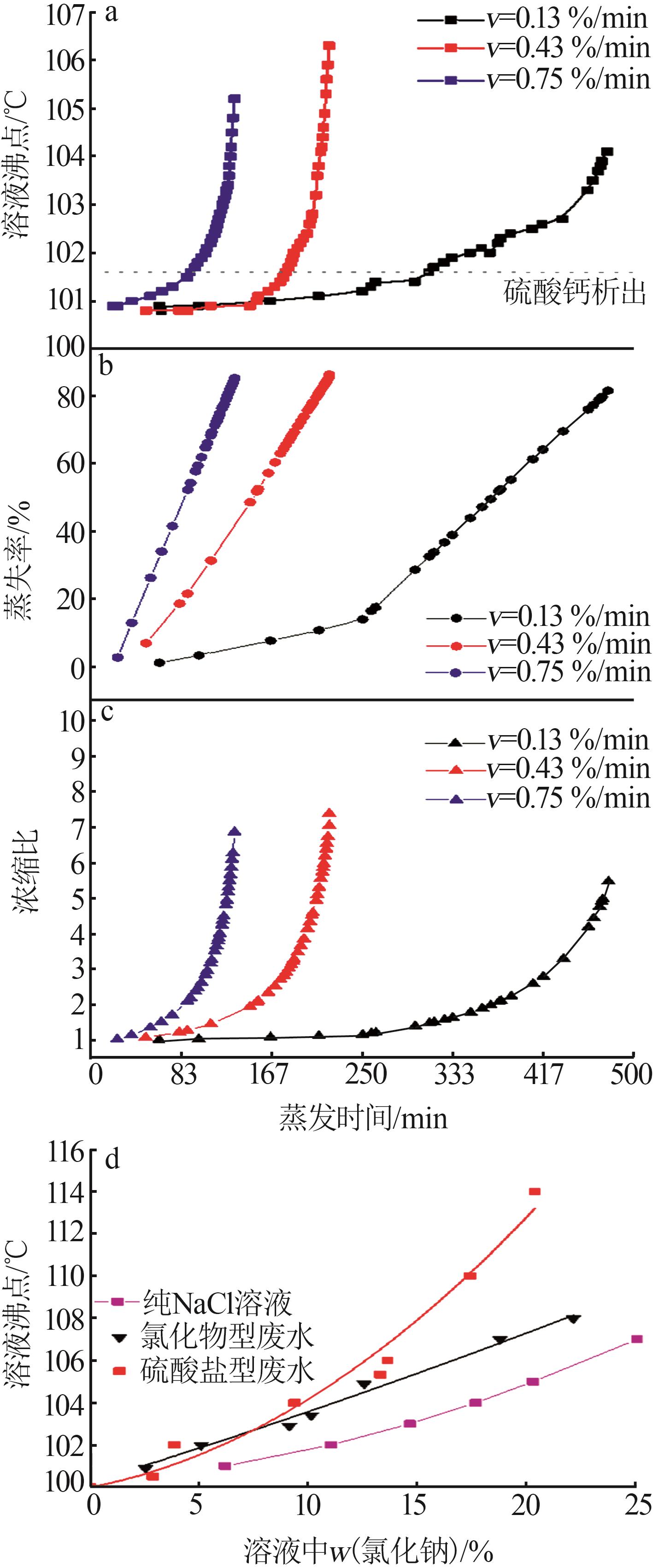
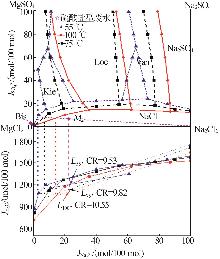
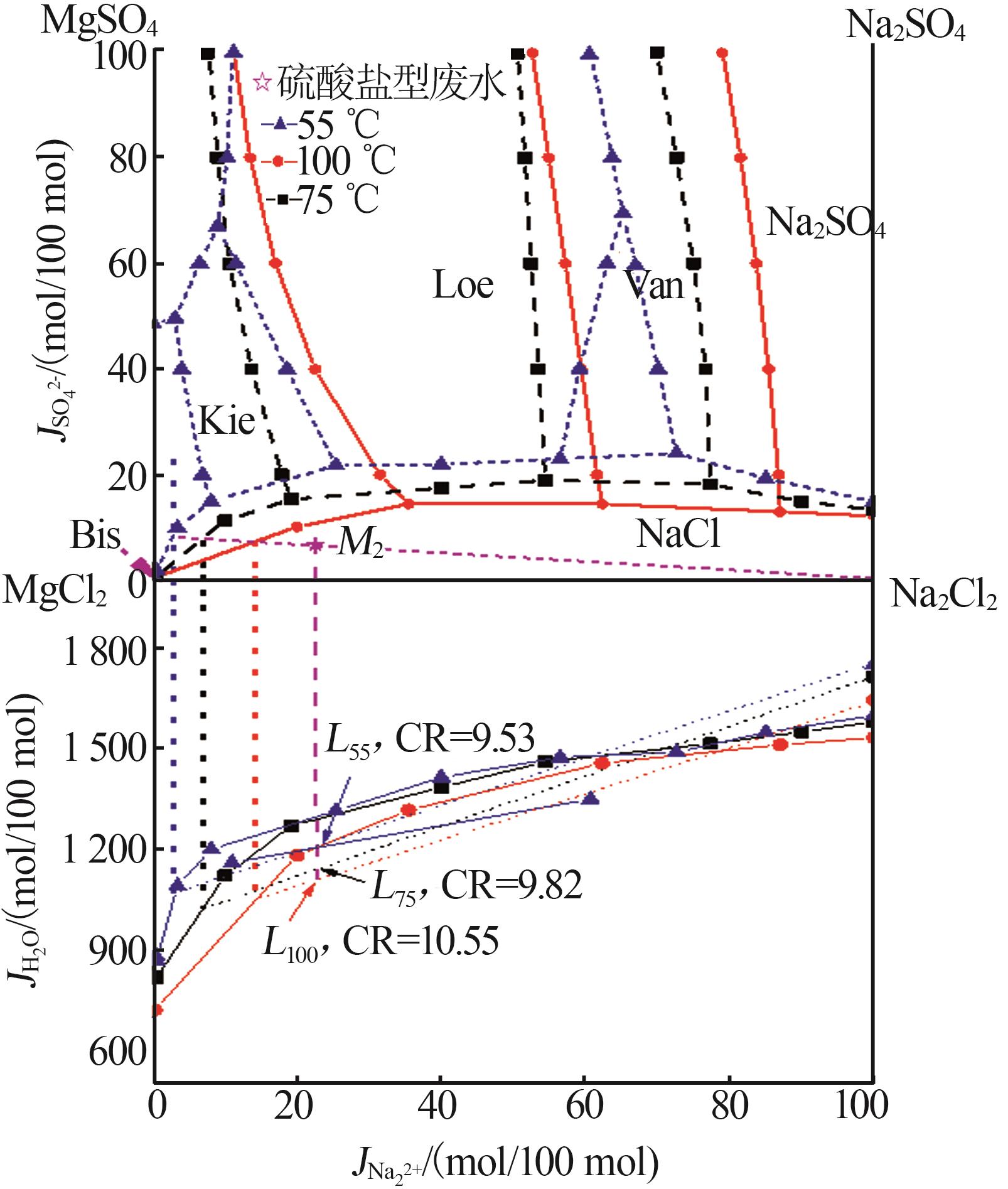
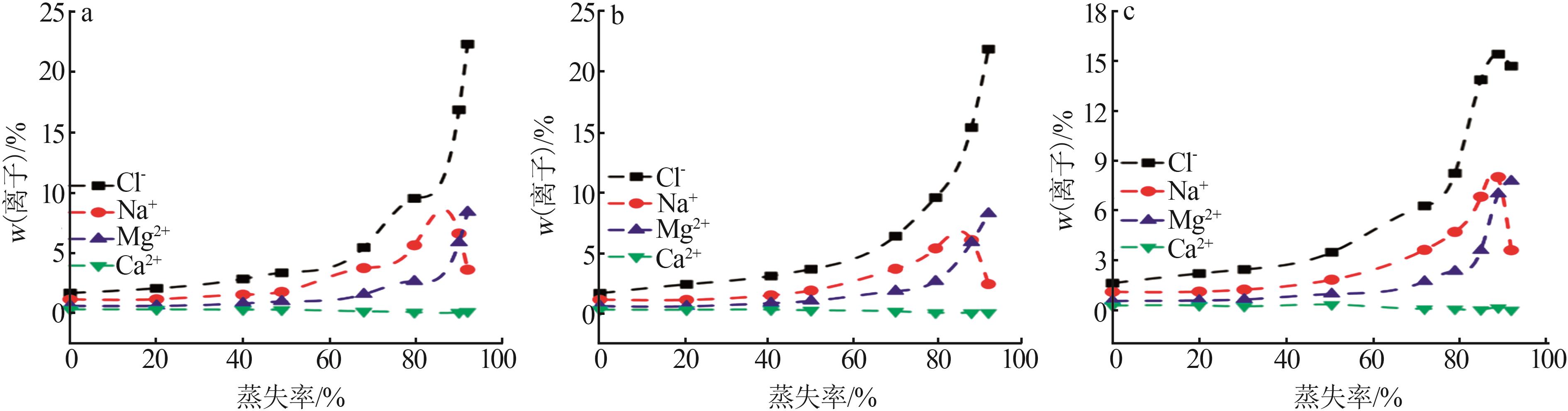
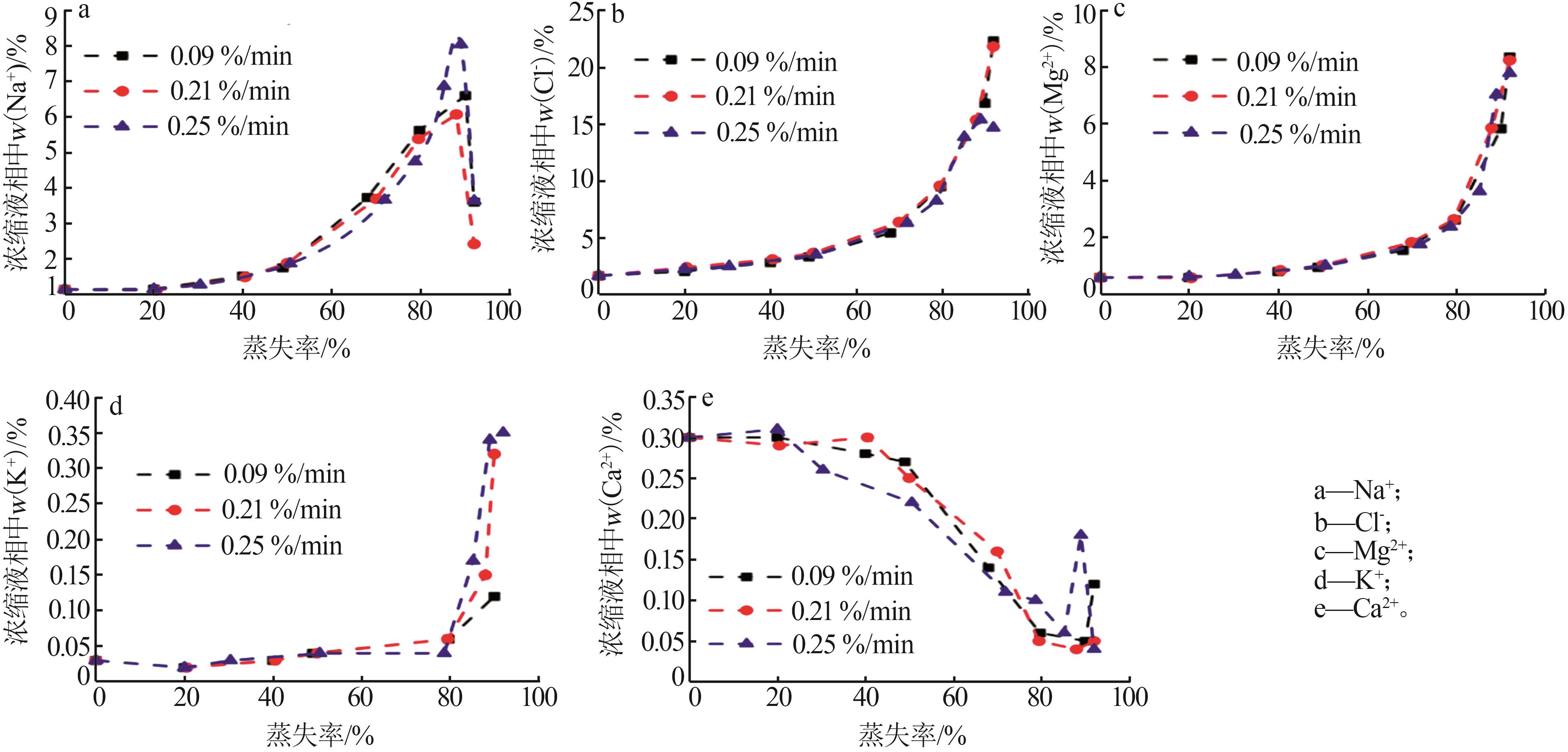
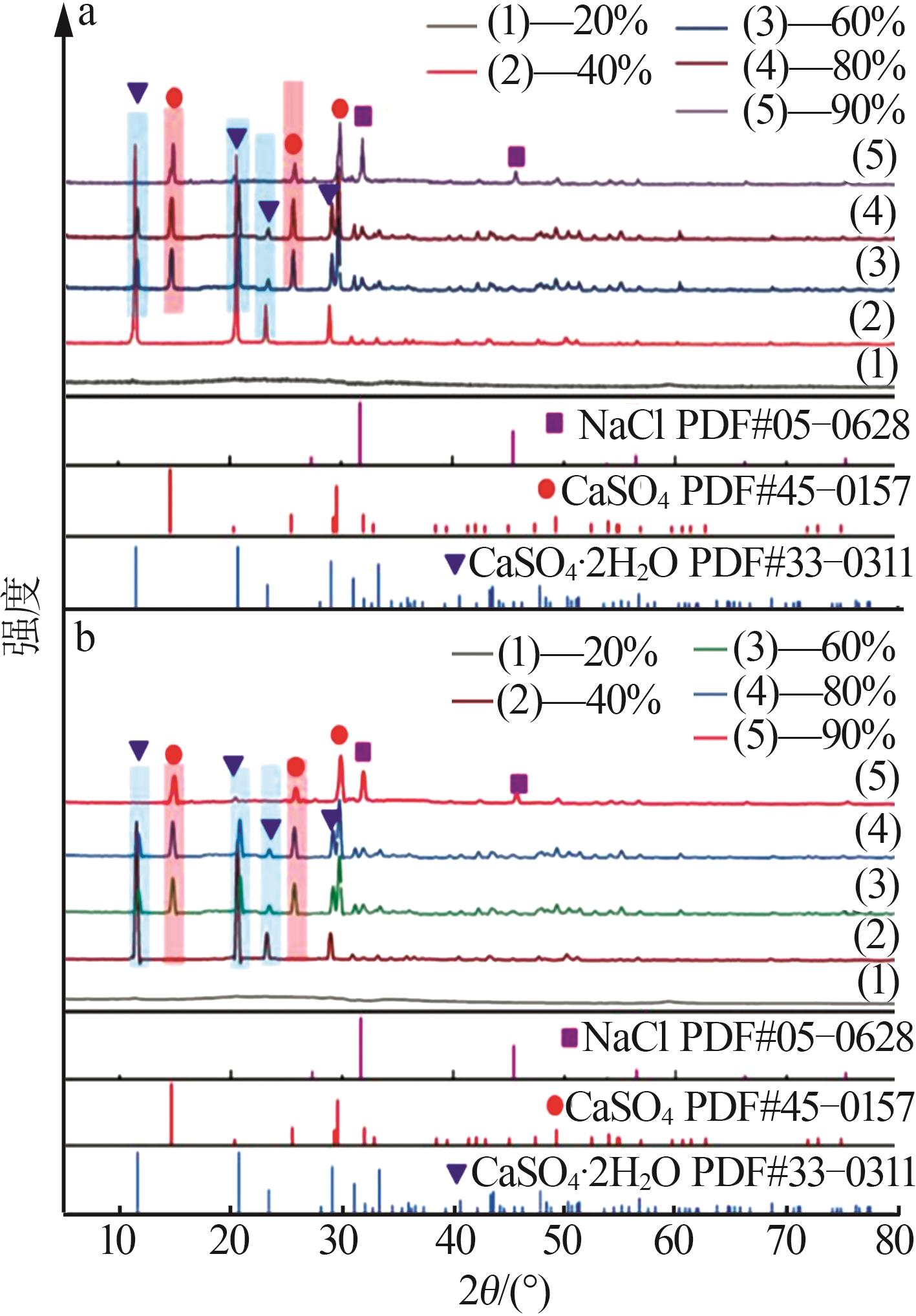
Table 1
Compositions of wastewater"
| 样品 | 质量浓度/(mg·L-1) | 浓度/(mol·L-1) | pH | 密度/(g·L-1) | 总溶解 固体(TDS)/% | 悬浮物(SS)/% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Ca2+ | Mg2+ | Na+ | SO42- | Cl- | K+ | Ca2+ | Mg2+ | Na+ | SO42- | Cl- | ||||||
| 氯化物型废水 | 1 490 | 3 660 | 2 764 | 10 060 | 2 120 | 19 140 | 0.038 1 | 0.091 3 | 0.113 7 | 0.437 6 | 0.022 1 | 0.539 9 | 5.89 | 1 020 | 4.57 | 1.11 | |
| 硫酸盐型废水 | 1 700 | 970 | 4 620 | 6 640 | 4 130 | 18 410 | 0.043 5 | 0.024 2 | 0.190 0 | 0.288 8 | 0.043 0 | 0.519 3 | 5.79 | 1 028 | 4.11 | 0.82 | |
| [1] | 胡大龙,余耀宏,于胜利,等.燃煤电厂脱硫废水处理技术现状与发展[J].工业水处理,2023,43(2):43-52. |
| HU Dalong, YU Yaohong, YU Shengli,et al.Present situation and development of treatment technology for desulfurization wastewater in coal-fired power station[J].Industrial Water Treatment,2023,43(2):43-52. | |
| [2] | 李鹏.脱硫废水的零排放处理技术应用[J].电子技术,2022,51(6):224-225. |
| LI Peng.Application of zero-discharge treatment technology for desulfurization wastewater[J].Electronic Technology,2022,51(6):224-225. | |
| [3] | 张娟,曹兆军,史海红,等.燃煤电厂脱硫废水分型与浓缩过程的盐类析出规律[J].动力工程学报,2022,42(1):67-74. |
| ZHANG Juan, CAO Zhaojun, SHI Haihong,et al.Salt precipitation behaviors of classification and concentration process for desulfurization wastewater in coal-fired power stations[J].Journal of Chinese Society of Power Engineering,2022,42(1):67-74. | |
| [4] | 靳苏娜,吕瑞亮.湿法脱硫废水处理技术研究及应用进展[J].无机盐工业,2023,55(4):27-37. |
| JIN Suna, Ruiliang LÜ.Research and application progress of wet flue gas desulfurization wastewater treatment technology[J].Inorganic Chemicals Industry,2023,55(4):27-37. | |
| [5] | NI Tingting, WANG Yunzhong, HUANG Chenghui,et al.Limestone-gypsum wet flue gas desulfurization wastewater treatment[J].IOP Conference Series:Earth and Environmental Science,2021,651(4):042034. |
| [6] | HAN Xiaoqu, ZHANG Dan, YAN Junjie,et al.Process development of flue gas desulphurization wastewater treatment in coal-fired power plants towards zero liquid discharge:Energetic,economic and environmental analyses[J].Journal of Cleaner Production,2020,261:121144. |
| [7] | 詹凌霄,陈恒,赵宁,等.工业高盐废水液滴蒸发特性研究进展[J].水处理技术,2022,48(4):1-5. |
| ZHAN Lingxiao, CHEN Heng, ZHAO Ning,et al.Research progresses of evaporation characteristics of industrial wastewater droplets with high salinity[J].Technology of Water Treatment,2022,48(4):1-5. | |
| [8] | 刘静颖,贾阳杰,杨凤玲,等.燃煤电厂脱硫废水零排放预处理工艺研究进展[J].无机盐工业,2023,55(12):12-25. |
| LIU Jingying, JIA Yangjie, YANG Fengling,et al.Research progress of zero liquid discharge pretreatment process for flue gas desulfurization wastewater from coal-fired power plants[J].Inorganic Chemicals Industry,2023,55(12):12-25. | |
| [9] | 马双忱,于伟静,贾绍广,等.燃煤电厂脱硫废水处理技术研究与应用进展[J].化工进展,2016,35(1):255-262. |
| MA Shuangchen, YU Weijing, JIA Shaoguang,et al.Research and application progresses of flue gas desulfurization(FGD) wastewater treatment technologies in coal-fired plants[J].Chemical Industry and Engineering Progress,2016,35(1):255-262. | |
| [10] | 张娟,夏攀平,郝晴,等.燃煤电厂脱硫废水全浓缩过程析盐规律的模型化[J].盐科学与化工,2022,51(11):4-9. |
| ZHANG Juan, XIA Panping, HAO Qing,et al.Modeling salt precipitation behaviors in the whole concentration process of desulfurization wastewater from coal-fired power plant[J].Journal of Salt Science and Chemical Industry,2022,51(11):4-9. | |
| [11] | 高红龙,杨文生,高磊,等.低温三效蒸发工艺中脱硫废水蒸发特性研究[J].工业水处理,2024,44(1):132-137. |
| GAO Honglong, YANG Wensheng, GAO Lei,et al.Evaporation characteristics of desulfurization wastewater in low temperature three effect evaporation process[J].Industrial Water Treatment,2024,44(1):132-137. | |
| [12] | WANG Yurui, ZHAN Lingxiao, CHEN Heng,et al.Study on the evaporation performance of concentrated desulfurization wastewater and its products analysis[J].Journal of Water Process Engineering,2024,58:104862. |
| [13] | SHI Wenxiao, LIN Chen, CHEN Wei,et al.Environmental effect of current desulfurization technology on fly dust emission in China[J].Renewable and Sustainable Energy Reviews,2017,72:1-9. |
| [14] | JIANG Binfan, XIE Yulei, XIA Dehong,et al.A potential source for PM2.5:Analysis of fine particle generation mechanism in wet flue gas desulfurization system by modeling drying and breakage of slurry droplet[J].Environmental Pollution,2019,246:249- 256. |
| [15] | 谷东杰,刘倩.固体废物中氟离子、溴酸根、氯离子、亚硝酸根、溴离子、硝酸根、磷酸根、硫酸根的离子色谱法测定[J].山东化工,2020,49(8):114-117. |
| GU Dongjie, LIU Qian.Solid waste-determination of fluoride,bromate,chloride,nitrite,bromine,nitrate,phosphate,sulfate-ion chromatography method[J].Shandong Chemical Industry,2020,49(8):114-117. | |
| [16] | 张晓明.离子色谱法同时测定复方电解质眼内冲洗液中不同阳离子含量[J].应用化工,2019,48(3):736-739. |
| ZHANG Xiaoming.Simultaneous determination of different cations content of inorganic salt in compound electrolyte intraocular irrigating solution by ion chromatography[J].Applied Chemical Industry,2019,48(3):736-739. | |
| [17] | HAN Xiaoqu, YUAN Tianrun, ZHANG Dan,et al.Waste heat utilization from boiler exhaust gases for zero liquid discharge of desulphurization wastewater in coal-fired power plants:Thermodynamic and economic analysis[J].Journal of Cleaner Production,2021,308:127328. |
| [18] | 王可苗.Ca-Mg-K-Cl-H2O盐溶液体系中硫酸钙的结晶过程[D].武汉:武汉科技大学,2013. |
| WANG Kemiao.Crystallization process of calcium sulfate in the Ca-Mg-K-Cl-H2O salt solution system[D].Wuhan:Wuhan University of Science and Technology,2013. | |
| [19] | GLATER J, MURDIA K S, DOOLY R.Calcium sulfate hemihydrate scaling threshold enhancement by magnesium ion augmentation[J].Desalination,1974,14(2):197-207. |
| [20] | 邓天龙,周桓,陈侠.水盐体系相图及应用[M].北京:化学工业出版社,2013. |
| DENG Tianlong, ZHOU Huan, CHEN Xia.Phase diagrams of water-salt systems and their applications[M].Beijing:Chemical Industry Press,2013. | |
| [21] | FANG Jiamei, SHI Chengcheng, ZHANG Lin,et al.Kinetic characteristics of evaporative crystallization desalination of acidic high-salt wastewater[J].Chemical Engineering Research and Design,2022,187:129-139. |
| [22] | 赖富国,高国华,肖燕飞,等.氯-硫酸盐体系下硫酸钙溶解度相图的研究进展[J].无机盐工业,2018,50(8):16-21. |
| LAI Fuguo, GAO Guohua, XIAO Yanfei,et al.Research progress on solubility phase diagrams of calcium sulfate in chloride-sulfate solutions[J].Inorganic Chemicals Industry,2018,50(8):16-21. |
| [1] | RAO Jiahuan, WEI Jiazhan, DENG Lian, JING Daiyan. Research progress on preparation of calcium sulfate whisker from industrial by⁃product gypsum under atmospheric pressure [J]. Inorganic Chemicals Industry, 2025, 57(5): 46-54. |
| [2] | LI Xuelian, TANG Zihan, XU Jie, LI Xiong. Study on performance of calcium sulfate whisker/SBS composite modified asphalt [J]. Inorganic Chemicals Industry, 2024, 56(9): 82-89. |
| [3] | DING Xiaojiang, WU Yanni, LI Boyun, HUANG Youliang. Research on preparation of carnallite concentrate by pretreatment combined with reverse flotation [J]. Inorganic Chemicals Industry, 2024, 56(12): 113-119. |
| [4] | LI Shan, ZHANG Daping, YANG Xiushan, ZHAO Qiang, KONG Xingjian. Study on transfer laws of phosphorus and fluorine impurities during producing calcium sulfide through sulfur reduction decomposition of phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(10): 110-117. |
| [5] | JIN Suna, LÜ Ruiliang. Research and application progress of wet flue gas desulfurization wastewater treatment technology [J]. Inorganic Chemicals Industry, 2023, 55(4): 27-37. |
| [6] | LIU Jingying, JIA Yangjie, YANG Fengling, REN Lei, LI Pengfei, WANG Fei, CHENG Fangqin. Research progress of zero liquid discharge pretreatment process for flue gas desulfurization wastewater from coal-fired power plants [J]. Inorganic Chemicals Industry, 2023, 55(12): 12-25. |
| [7] | HE Lei,ZHU Ganyu,ZHENG Guangming,WU Wenfen,ZhANG Jianbo,LI Fang,LI Huiquan,CHEN Wen. Study on crystallization process and mechanism of phosphogypsum in wet process phosphoric acid system [J]. Inorganic Chemicals Industry, 2022, 54(7): 110-116. |
| [8] | MA Lei,SHENG Yu,ZHOU Junhong,YANG Yujun,LUO Hui,PAN Chunying,WANG Guo. Study on new process of comprehensive utilization and separation of sulfur and calcium of titanium gypsum [J]. Inorganic Chemicals Industry, 2022, 54(7): 124-128. |
| [9] | LI Ming,LI Zhili,ZHANG Zeqiang,HE Dongsheng,TANG Yuan,CHEN Fei. Preparation of calcium sulfate whiskers assisted by mixed sodium fatty acid [J]. Inorganic Chemicals Industry, 2022, 54(6): 66-72. |
| [10] | MAO Xinyu,WANG Yubin,WANG Wenwen,LI Shuqin,MA Xiaoxiao. Effect of alternating external magnetic field on the dissolution behavior of calcium sulfate scale and its mechanism [J]. Inorganic Chemicals Industry, 2022, 54(3): 97-101. |
| [11] | QI Yuanhao,WU Jinxiu,LIU Zhaogang,HU Yanhong,FENG Fushan,LI Jianfei,WANG Xin,LIU Conglin. Preparation and characterization of anhydrous calcium sulfate whisker [J]. Inorganic Chemicals Industry, 2022, 54(10): 109-115. |
| [12] | MU Hong,ZHOU Guiyun,HU Haiyang. Study on process of preparing calcium sulfate whiskers from phosphate rock acid hydrolysis solution [J]. Inorganic Chemicals Industry, 2022, 54(1): 51-55. |
| [13] | LI Zheng,HU Meng,DOU Yan,CUI Peng,SHEN Hao,ZHENG Zhiyin,LIU Rong. Effect of sodium oxalate on stability of calcium sulfate hemihydrate crystal [J]. Inorganic Chemicals Industry, 2022, 54(1): 62-65. |
| [14] | Zhang Lizhen,Lü Zihu,Zhang Yongxing,Wu Zhaoyang,Zhang Xiufeng,Tan Xiumin. Experimental study on improving quality and reducing impurity of phosphogypsum [J]. Inorganic Chemicals Industry, 2021, 53(6): 171-173. |
| [15] | Xu Wei,Li Mei,Zhang Dongliang,Gao Kai,Li Jianfei. Study on preparation of calcium sulfate whisker by atmospheric pressure acidification using rare earth gypsum [J]. Inorganic Chemicals Industry, 2020, 52(8): 66-71. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||