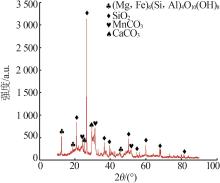
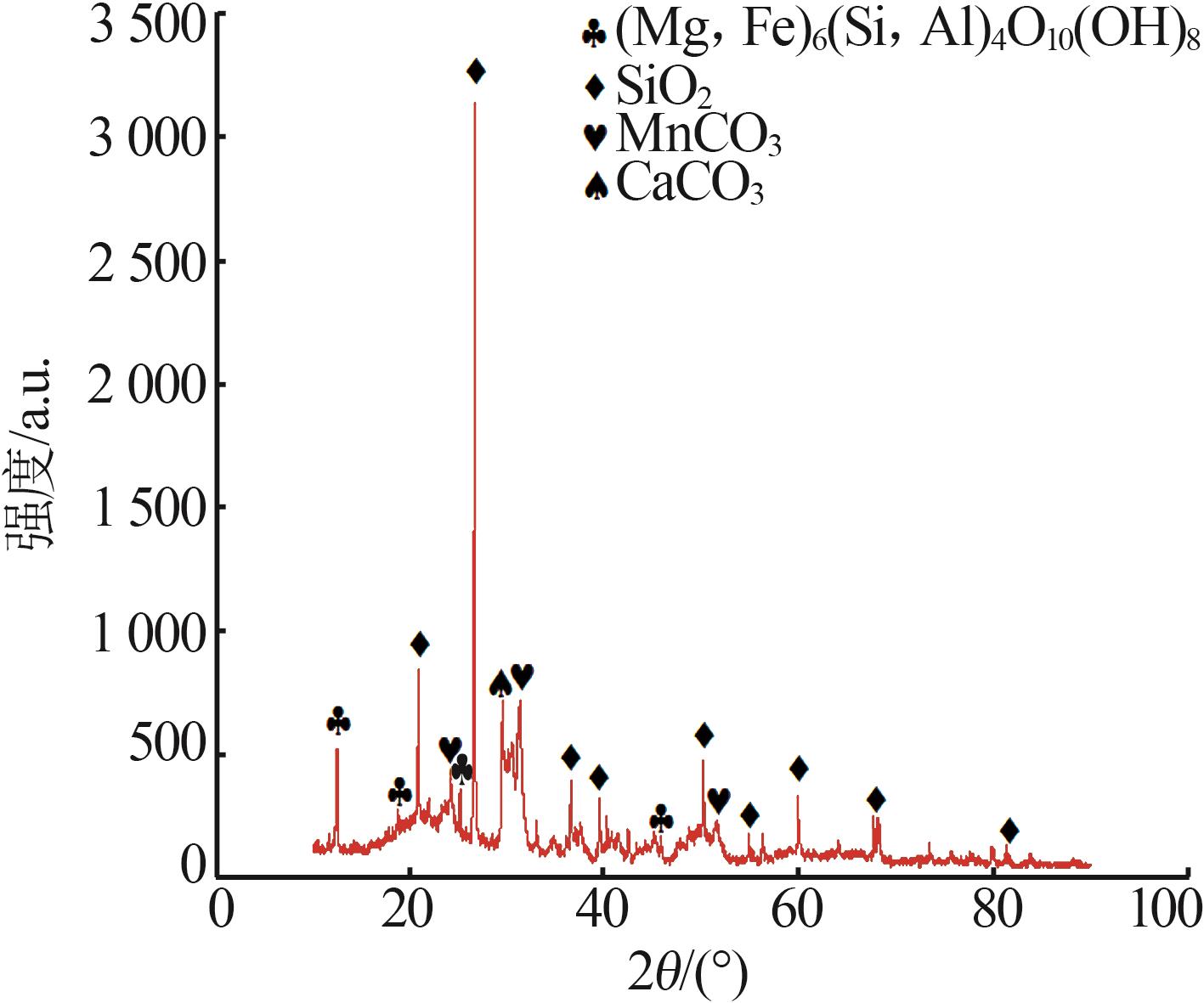
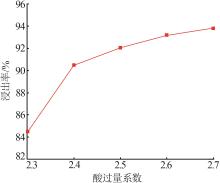
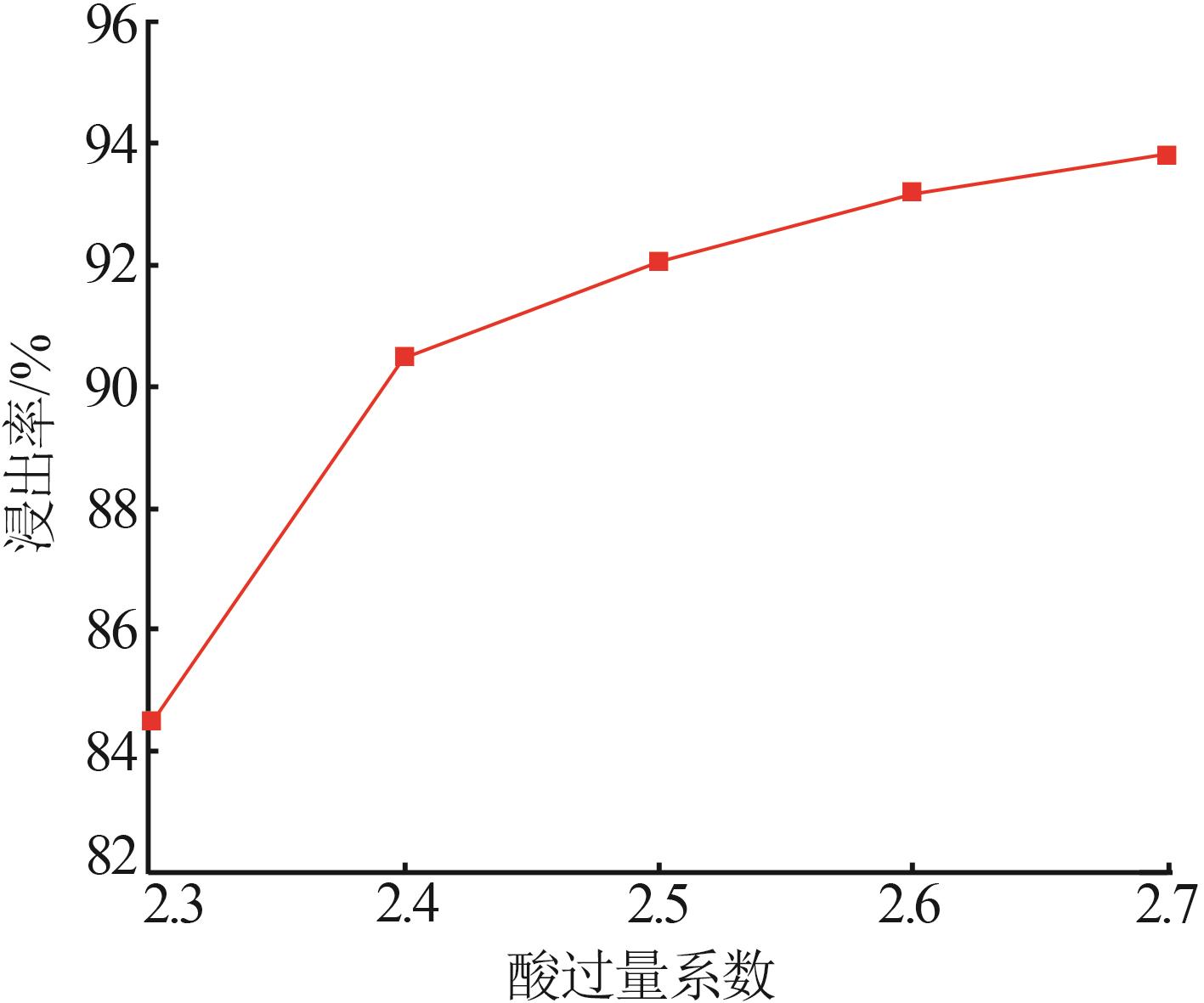
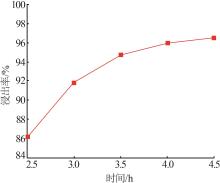
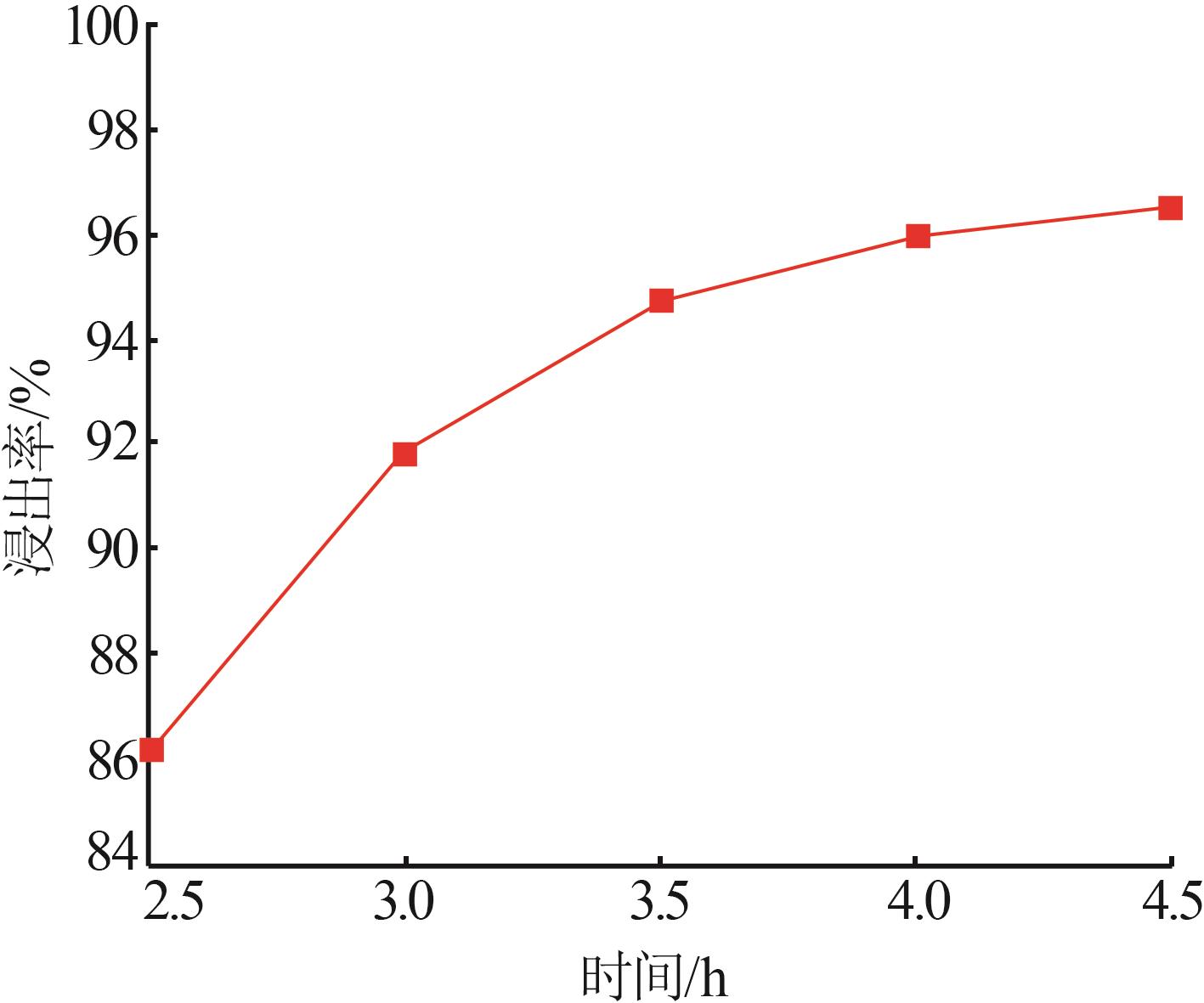
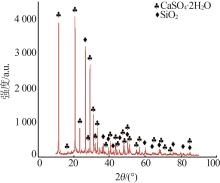
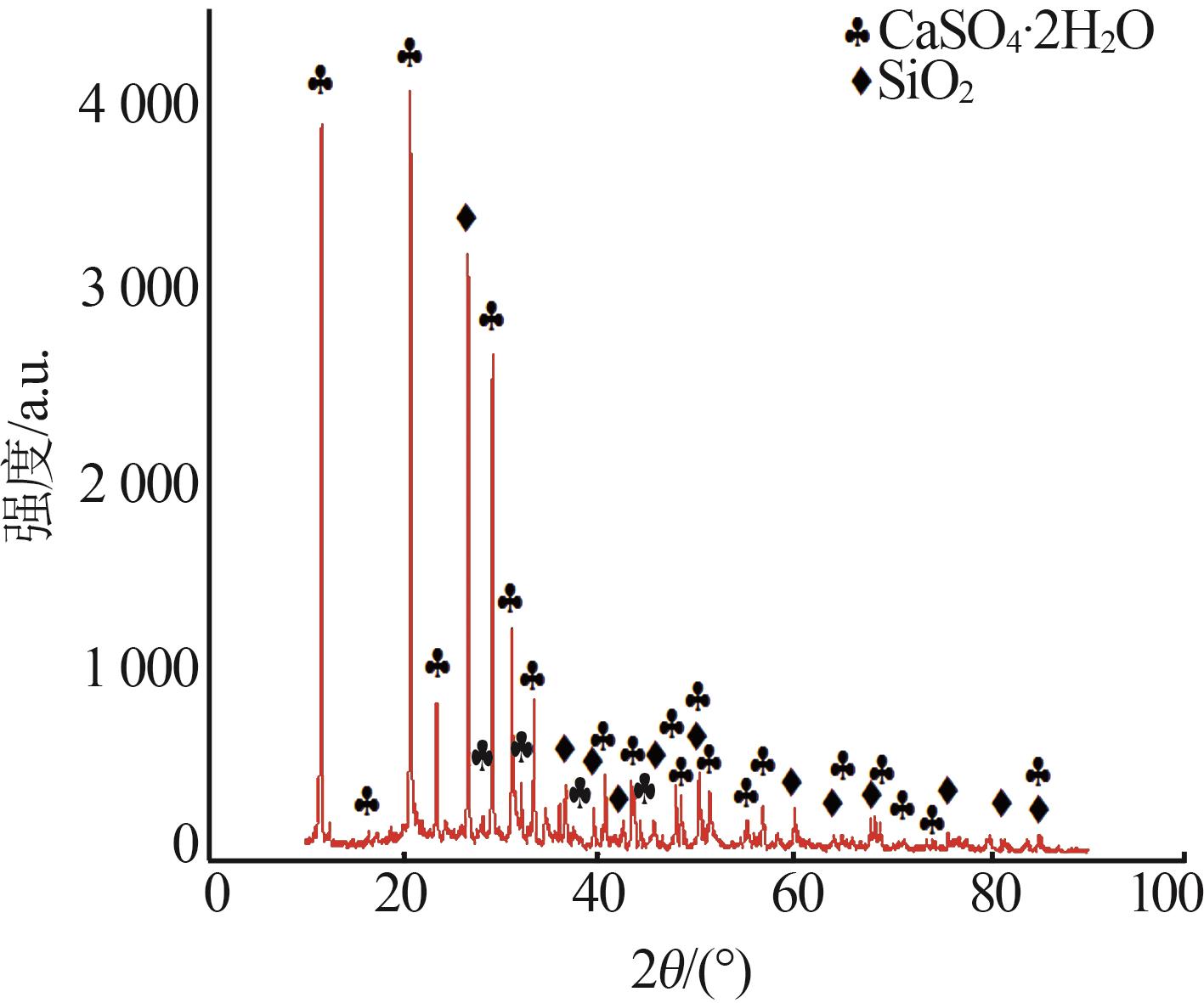
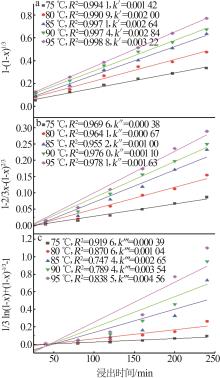
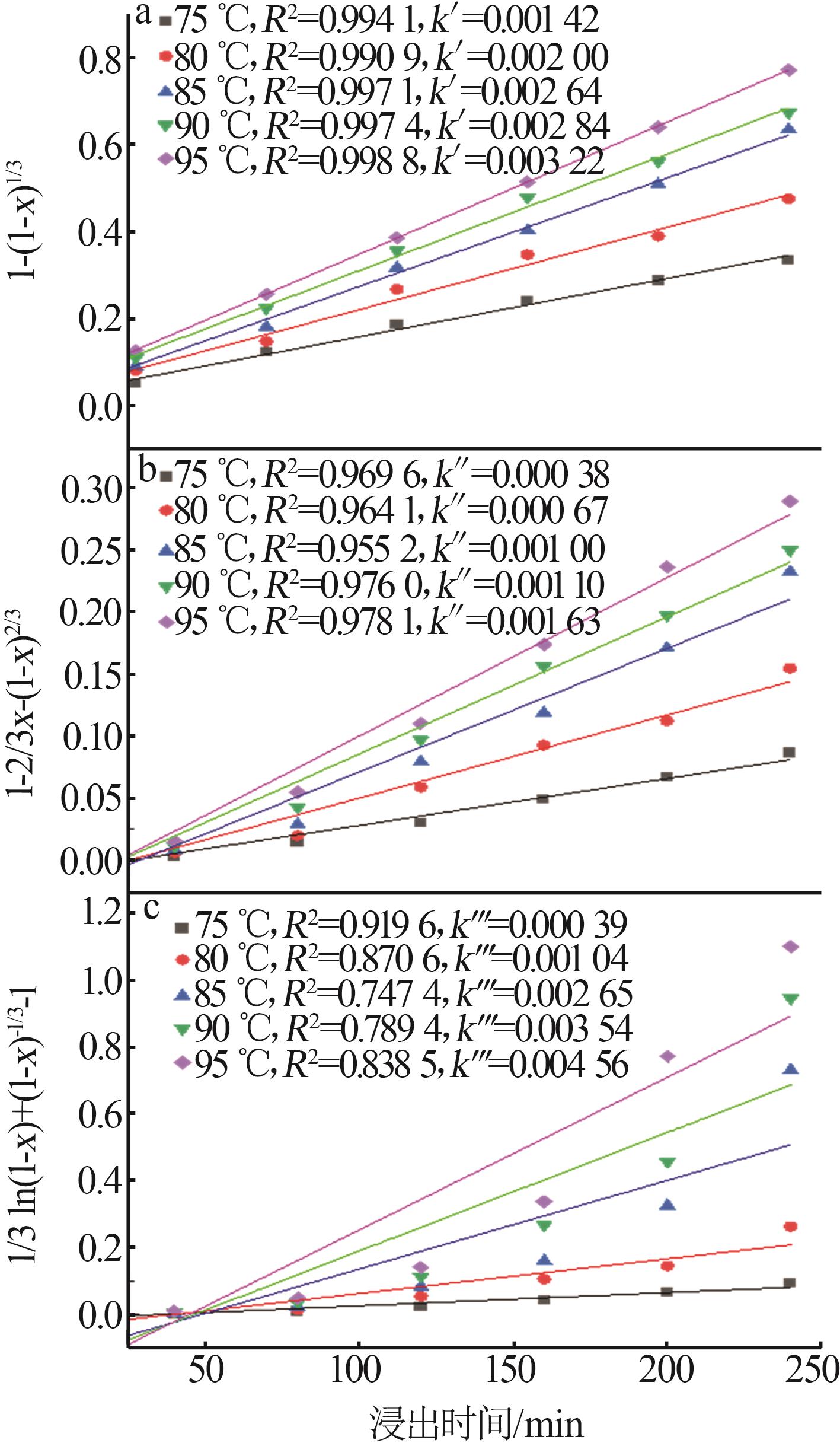
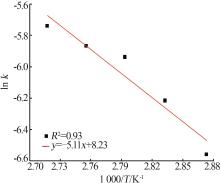
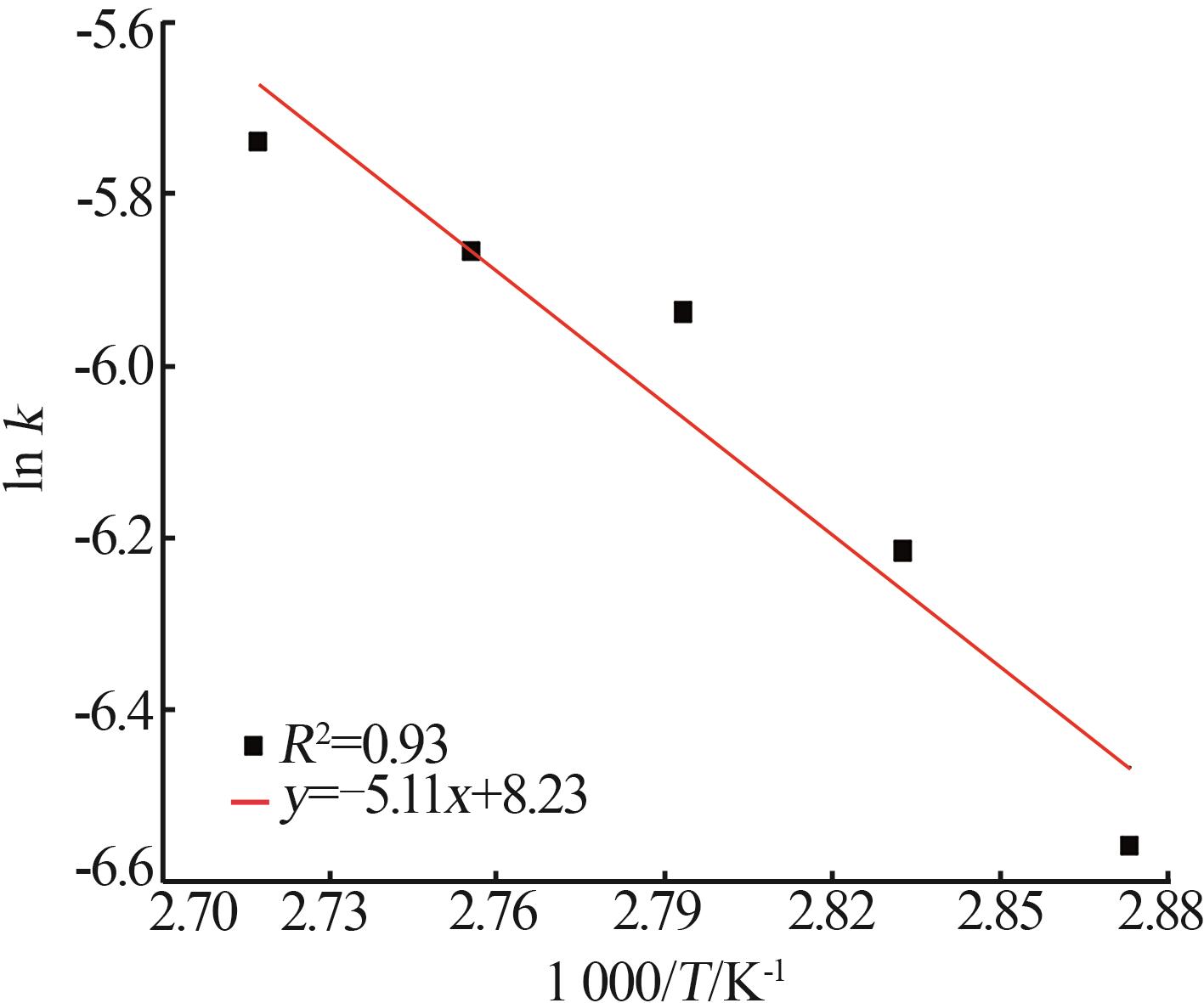
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (1): 51-57.doi: 10.19964/j.issn.1006-4990.2024-0106
• Research & Development • Previous Articles Next Articles
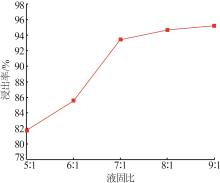
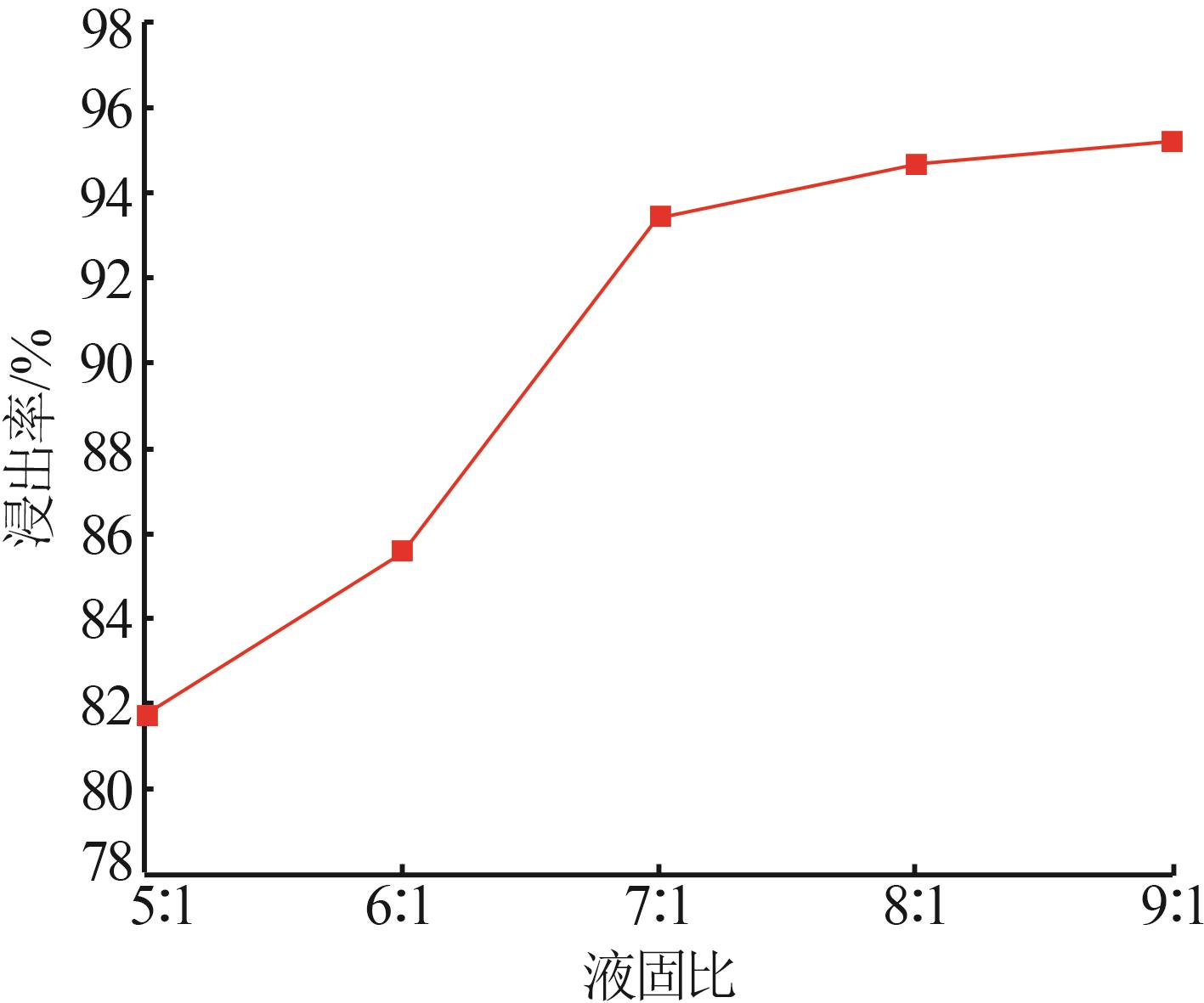
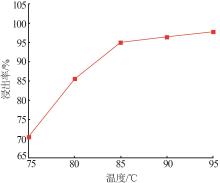
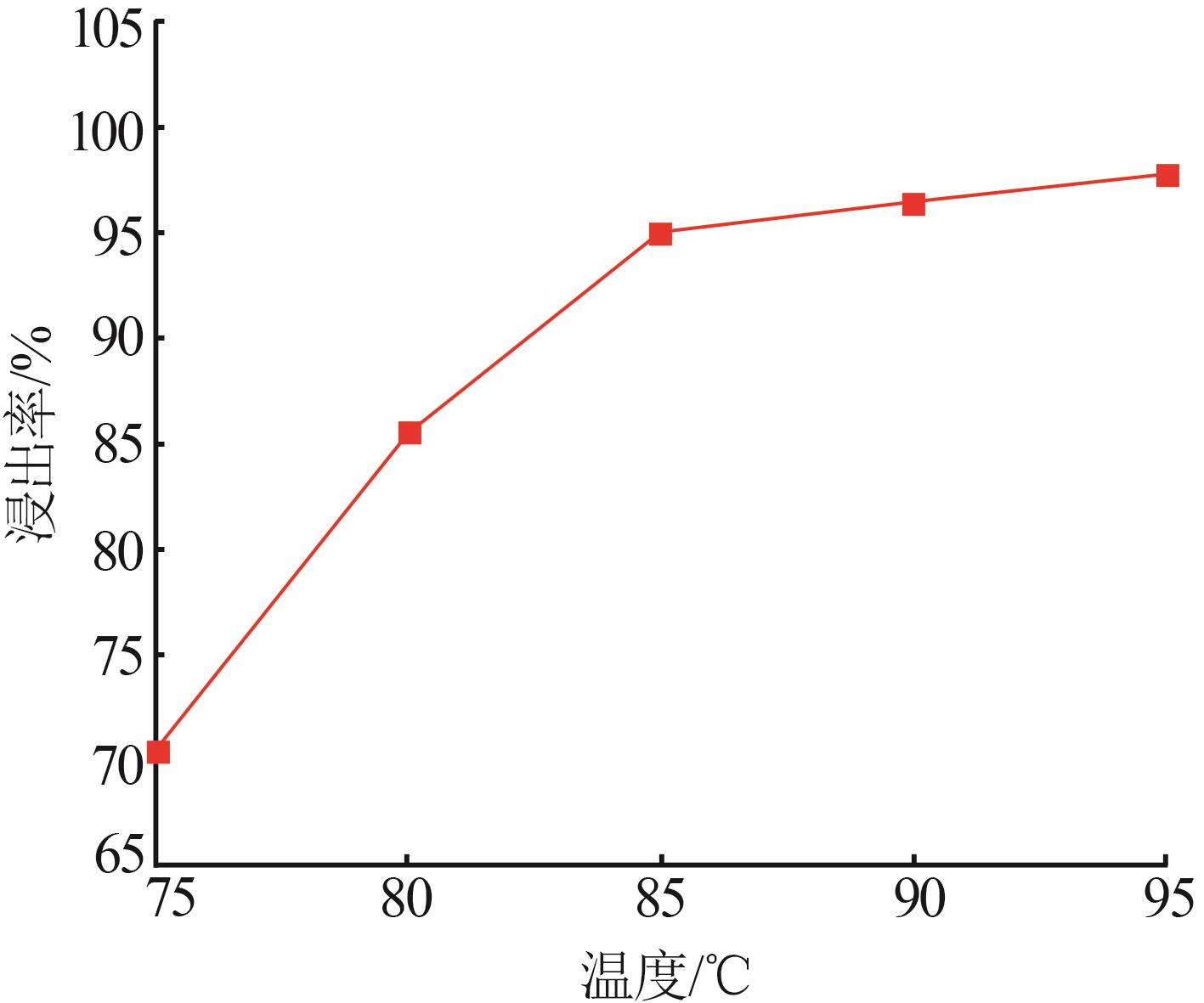
Study on leaching and kinetics of rhododengite in Jingxi area of Guangxi
ZHENG Kexin1,2( ), WANG Haifeng1,2,3(
), WANG Haifeng1,2,3( ), WANG Jiawei1,2,3, ZHOU Xingjie1,2, PEI Zhengqing1,2, LU Ju1,2, MA Dehua1,2
), WANG Jiawei1,2,3, ZHOU Xingjie1,2, PEI Zhengqing1,2, LU Ju1,2, MA Dehua1,2
- 1.College of materials and metallurgy,Guizhou University,Guiyang 550025,China
2.Guizhou Key Laboratory of metallurgical engineering and process energy conservation,Guiyang 550025,China
3.Engineering Technology and Research Center of Manganese Material for Battery,Tongren 554300,China
-
Received:2024-02-28Online:2025-01-10Published:2025-01-24 -
Contact:WANG Haifeng E-mail:zhengkexin@163.com;mm.hfwang@163.com
CLC Number:
Cite this article
ZHENG Kexin, WANG Haifeng, WANG Jiawei, ZHOU Xingjie, PEI Zhengqing, LU Ju, MA Dehua. Study on leaching and kinetics of rhododengite in Jingxi area of Guangxi[J]. Inorganic Chemicals Industry, 2025, 57(1): 51-57.
share this article
| 1 | 王松.菱锰矿浸出液净化制备电池级Mn3O4的调控机制研究[D].贵阳:贵州大学,2023. |
| WANG Song.Study on the regulation mechanism of purification of rhodochrosite leaching solution to prepare battery-grade Mn3O4 [D].Guiyang:Guizhou University,2023. | |
| 2 | 任辉,刘敏,王自国,等.我国锰矿资源及产业链安全保障问题研究[J].中国工程科学,2022,24(3):20-28. |
| REN Hui, LIU Min, WANG Ziguo,et al.Security of manganese resources and industrial chain in China[J].Strategic Study of CAE,2022,24(3):20-28. | |
| 3 | SHI Guangyao, WANG Xueqiu, WANG Wei,et al.Nation-wide concentration and spatial distribution of manganese with links to manganese mineralization in China[J].Journal of Geochemical Exploration,2023,244:107130. |
| 4 | 吕子虎,李成禄,赵登魁,等.菱锰矿选冶技术研究现状[J].中国矿业,2023,32(7):105-111. |
| Zihu LÜ, LI Chenglu, ZHAO Dengkui,et al.Research status of dressing-metallurgy technology of rhodochrosite ore[J].China Mining Magazine,2023,32(7):105-111. | |
| 5 | 唐道文,董雄文,李军旗,等.软锰矿、菱锰矿联合脱硫制备电池用硫酸锰的工业化试验探讨[J].有色金属科学与工程,2023,14(4):454-459. |
| TANG Daowen, DONG Xiongwen, LI Junqi,et al.Industrial experiment study on preparation of battery grade manganese sulfate by combined desulfurization of pyrolusite and rhodochrosite[J].Nonferrous Metals Science and Engineering,2023,14(4):454-459. | |
| 6 | 郑宇,李炳震,刘长根,等.软锰矿-硫铁矿协同浸出实验研究[J].矿冶工程,2022,42(5):103-105,110. |
| ZHENG Yu, LI Bingzhen, LIU Changgen,et al.Simultaneous leaching of manganese from pyrolusite and pyrite[J].Mining and Metallurgical Engineering,2022,42(5):103-105,110. | |
| 7 | 邹廷信,聂程,毛拥军.某进口软锰矿还原焙烧、浸出实验研究[J].矿冶工程,2022,42(3):115-117. |
| ZOU Tingxin, NIE Cheng, MAO Yongjun.Experimental study on reduction roasting and leaching of imported pyrolusite[J].Mining and Metallurgical Engineering,2022,42(3):115-117. | |
| 8 | 秦志祥,杨婷,卢友志,等.响应曲面法优化速生桉尾料还原浸出软锰矿试验研究[J].中国锰业,2021,39(4):22-25. |
| QIN Zhixiang, YANG Ting, LU Youzhi,et al.Optimization of reductive leaching of pyrolusite from fast-growing eucalyptus tail residue by response surface methodology[J].China Manganese Industry,2021,39(4):22-25. | |
| 9 | PRASETYO E, PURWANINGSIH E, ASTUTI W.Selective-reductive leaching of manganese from low-grade manganese ore using tannic acid as reductant[J].Mining,Metallurgy & Exploration,2019,36(5):1003-1012. |
| 10 | 卢友志,苏李敏,赵义.单宁酸-硫酸体系浸出氧化锰矿中锰的研究[J].无机盐工业,2022,54(6):84-89. |
| LU Youzhi, SU Limin, ZHAO Yi.Study on leaching of manganese from manganese dioxide ore with tannic acid-sulfuric acid system[J].Inorganic Chemicals Industry,2022,54(6):84-89. | |
| 11 | MORO K, HAUBRICH F, MARTIN M,et al.Reductive leaching behaviour of manganese and cobalt phases in laterite and manganese ores[J].Hydrometallurgy,2023,220:106101. |
| 12 | 卢友志,王雅梦,赵义.核桃壳还原浸出软锰矿的动力学[J].有色金属(冶炼部分),2021(12):28-34. |
| LU Youzhi, WANG Yameng, ZHAO Yi.Reductive leaching kinetics of pyrolusite with walnut shell[J].Nonferrous Metals(Extractive Metallurgy),2021(12):28-34. | |
| 13 | YANG Peng, LIANG Xiaoping, WU Chengbo,et al.Improve manganese leaching efficiency by adding different complexants during ammonia leaching of low-grade rhodochrosite[J].Minerals Engineering,2022,188:107834. |
| 14 | ZENG Xiangfei, SHU Jiancheng, CHEN Mengjun,et al.A cost-effective method for enhancing manganese leaching from rhodochrosite caused by surfactant-regulated crystal growth of CaSO4·2H2O[J].Hydrometallurgy,2022,211:105870. |
| 15 | YANG Peng, LIANG Xiaoping, WU Chengbo,et al.Study on adding ammonium hydrogen fluoride to improve manganese leaching efficiency of ammonia leaching low-grade rhodochrosite[J].Metals,2021,11(9):1496. |
| 16 | DE OLIVEIRA T, GUÉGAN R, THIEBAULT T,et al.Adsorption of diclofenac onto organoclays:Effects of surfactant and environmental(pH and temperature) conditions[J].Journal of Hazardous Materials,2017,323:558-566. |
| 17 | AI Chunming, SUN Pingping, WU Aixiang,et al.Accelerating leaching of copper ore with surfactant and the analysis of reaction kinetics[J].International Journal of Minerals,Metallurgy,and Materials,2019,26(3):274-281. |
| 18 | YIN Shenghua, WANG Leiming, WU Aixiang,et al.Enhancement of copper recovery by acid leaching of high-mud copper oxides:A case study at yangla copper mine,China[J].Journal of Cleaner Production,2018,202:321-331. |
| 19 | 曾祥菲.基于表界面强化的菱锰矿浸出研究[D].绵阳:西南科技大学,2022. |
| ZENG Xiangfei.Rhodochrosite leaching based on surface-interface enhancement[D].Mianyang:Southwest University of Science and Technology,2022. | |
| 20 | PRASETYO E, PURWANINGSIH E, ASTUTI W.Selective-reductive leaching of manganese from low-grade manganese ore using tannic acid as reductant[J].Mining,Metallurgy & Exploration,2019,36(5):1003-1012. |
| 21 | OSALI M, AHANI F, KHODAEI H,et al.Reductive leaching and recovery of nano-crystalline MnO2 from low-grade pyrolusite ore[J].Journal of Sustainable Metallurgy,2023,9(3):1268-1278. |
| 22 | SINHA M K, JORDAAN R, PURCELL W.Hydrometallurgical recovery of manganese from ferruginous manganese ore by reductive-acid leaching with sodium dithionite[J].Journal of Sustainable Metallurgy,2022,8(2):783-794. |
| 23 | 李昌新,李秋月,喻源,等.以高硫锰矿制备电池用硫酸锰的净化除杂工艺研究[J].无机盐工业,2018,50(7):27-32. |
| LI Changxin, LI Qiuyue, YU Yuan,et al.Purification process of electronic grade manganese sulfate prepared by high-sulfur manganese ores[J].Inorganic Chemicals Industry,2018,50(7):27-32. | |
| 24 | DU Jinjia, ZHANG Yanqiong, LU Jiajia,et al.Mechanism of enhanced enrichment manganese from manganese ore-pyrite under microwave heating:Process optimization and kinetic studies[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2023,656:130534. |
| 25 | LIU Dongjie, GAO Lei, CHEN Guo,et al.Enhancement effects of distiller′s dried grains as reducing agents on the kinetics and leaching of pyrolusite from manganese ore[J].Journal of Materials Research and Technology,2022,19:4270-4281. |
| [1] | TAN Shanyi, WEN Huizi, HE Shuyu, ZHANG Liwen, CHEN Shaohua, XI Benjun. Study on leaching behavior and kinetics of phosphorus from phosphogypsum [J]. Inorganic Chemicals Industry, 2025, 57(2): 105-112. |
| [2] | YAN Xin, LIU Hailu, LIU Baolin, LIU Yi, LIU Yanyang. Research on key technologies and mechanisms of green nano calcium carbonate production [J]. Inorganic Chemicals Industry, 2025, 57(1): 71-76. |
| [3] | TANG Dongwu, YE Changwen, DENG Jie, AO Fang. Study on leaching rate of calcium and magnesium from phosphorus tailings based on thermodynamic analysis and response surface method [J]. Inorganic Chemicals Industry, 2024, 56(9): 98-106. |
| [4] | SU Hang, SONG Jitian, HUANG Zhiqiang, DONG Qing, ZHANG Yaxiong. Study on crystallization kinetics of manganese sulfate monohydrate in H2SO4-H2O binary system [J]. Inorganic Chemicals Industry, 2024, 56(8): 40-46. |
| [5] | XU Zijian, WANG Yu, LIU Jingyong, ZHU Chuanghai, CHEN Zhibin, HUANG Shengzheng, XIE Wuming, SUN Shuiyu. Study on defluorination process of secondary aluminum ash wet process and conversion behavior of fluoride [J]. Inorganic Chemicals Industry, 2024, 56(6): 109-118. |
| [6] | REN Xuechang, FENG Hao. Study on dissolution process of sintered clinker by aluminum ash alkali method with ultrasound irritation [J]. Inorganic Chemicals Industry, 2024, 56(6): 119-126. |
| [7] | LU Ju, WANG Jiawei, WANG Haifeng, PEI Zhengqing, ZHOU Xingjie, ZHENG Kexin, MA Dehua. Study on purification of magnesium from manganese electrolyte by fluorine salt method [J]. Inorganic Chemicals Industry, 2024, 56(6): 40-45. |
| [8] | LIN Weizhi, FU Chengbing, HU Ping, YANG Kaixu, CAO Jianxin. Study on mineralogical characteristics and occurrence state of elements of rhodochrosite in Tongren area [J]. Inorganic Chemicals Industry, 2024, 56(6): 73-79. |
| [9] | DAI Hongtao, SHEN Fangfang, YANG Wentao. Experimental study on preparation of ceramic permeable pavement brick from waste incineration slag [J]. Inorganic Chemicals Industry, 2024, 56(5): 121-127. |
| [10] | FANG Xiaoning, KUANG Fei, LIU Chenglin. Study on extraction of potassium from K-feldspar by roasting-leaching of mixed salts [J]. Inorganic Chemicals Industry, 2024, 56(5): 53-57. |
| [11] | YU Zhou, HE Zhaoyi, TANG Liang, HE Sheng, XIAO Haixin, XIAO Yixun. Study on preparation and microscopic properties of typical sulfate solid waste composite cementitious materials [J]. Inorganic Chemicals Industry, 2024, 56(4): 90-97. |
| [12] | WANG Jianrui, ZHANG Song, ZHANG Jie. Study on new process for beneficiation phosphate of low-grade phosphate rock leaching via lactic acid [J]. Inorganic Chemicals Industry, 2024, 56(3): 56-63. |
| [13] | WANG Ruting, ZHAO Xiaorong, HUANG Xuquan, WANG Haojie, XUE Fei, CAI Jiawei. Research on preparation and early performance of mixed phase phosphogypsum-based cementing materials [J]. Inorganic Chemicals Industry, 2024, 56(3): 98-104. |
| [14] | HUANG Tao, HUANG Zili, XIAO Shuo, ZHENG Jiemiao, LIU Xiaofeng, WU Jilong. Experimental study on preparation of polyferric chloride from iron tailings acid leaching solution [J]. Inorganic Chemicals Industry, 2024, 56(2): 121-126. |
| [15] | XIA Guiying, YANG Liuchun, YUAN Zhiye. Study on direct leaching of rare earth elements from phosphogypsum with sulfuric acid [J]. Inorganic Chemicals Industry, 2024, 56(1): 107-113. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||