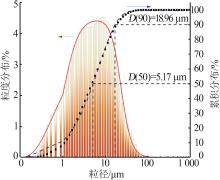
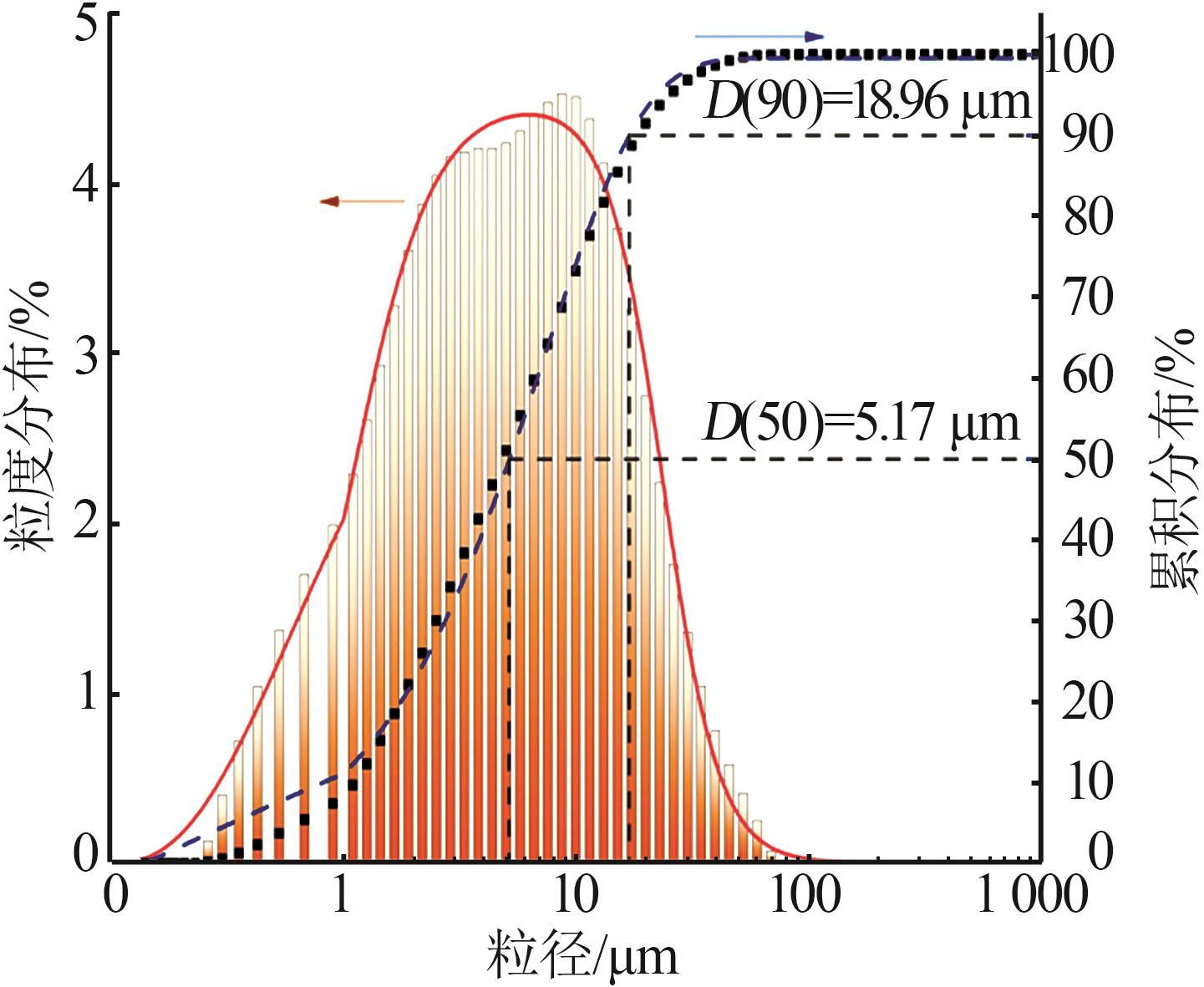
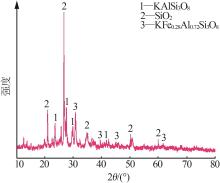
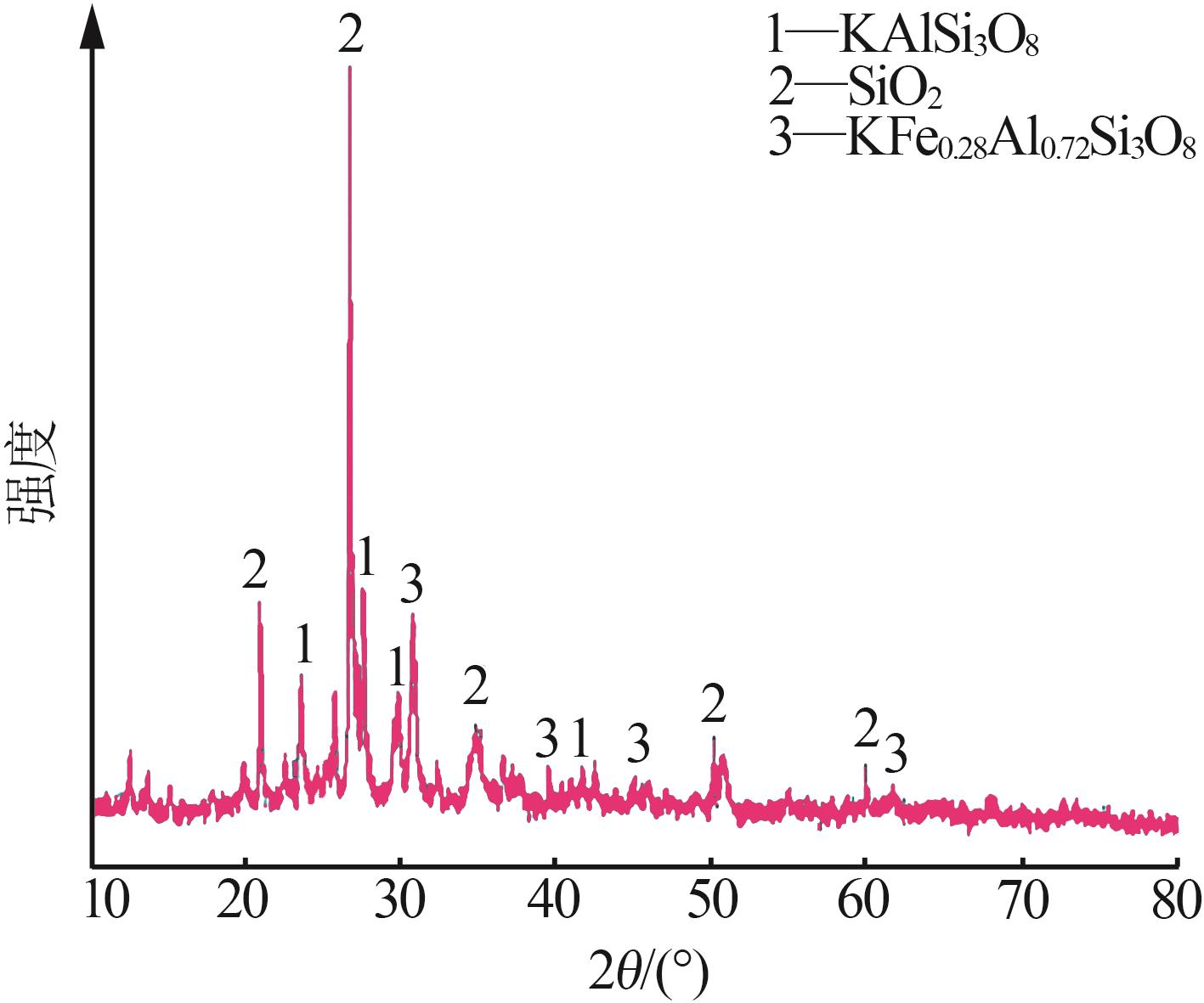
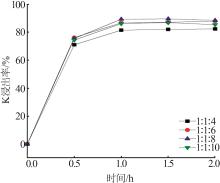
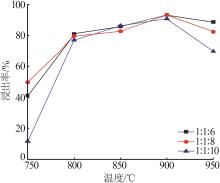
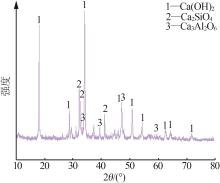
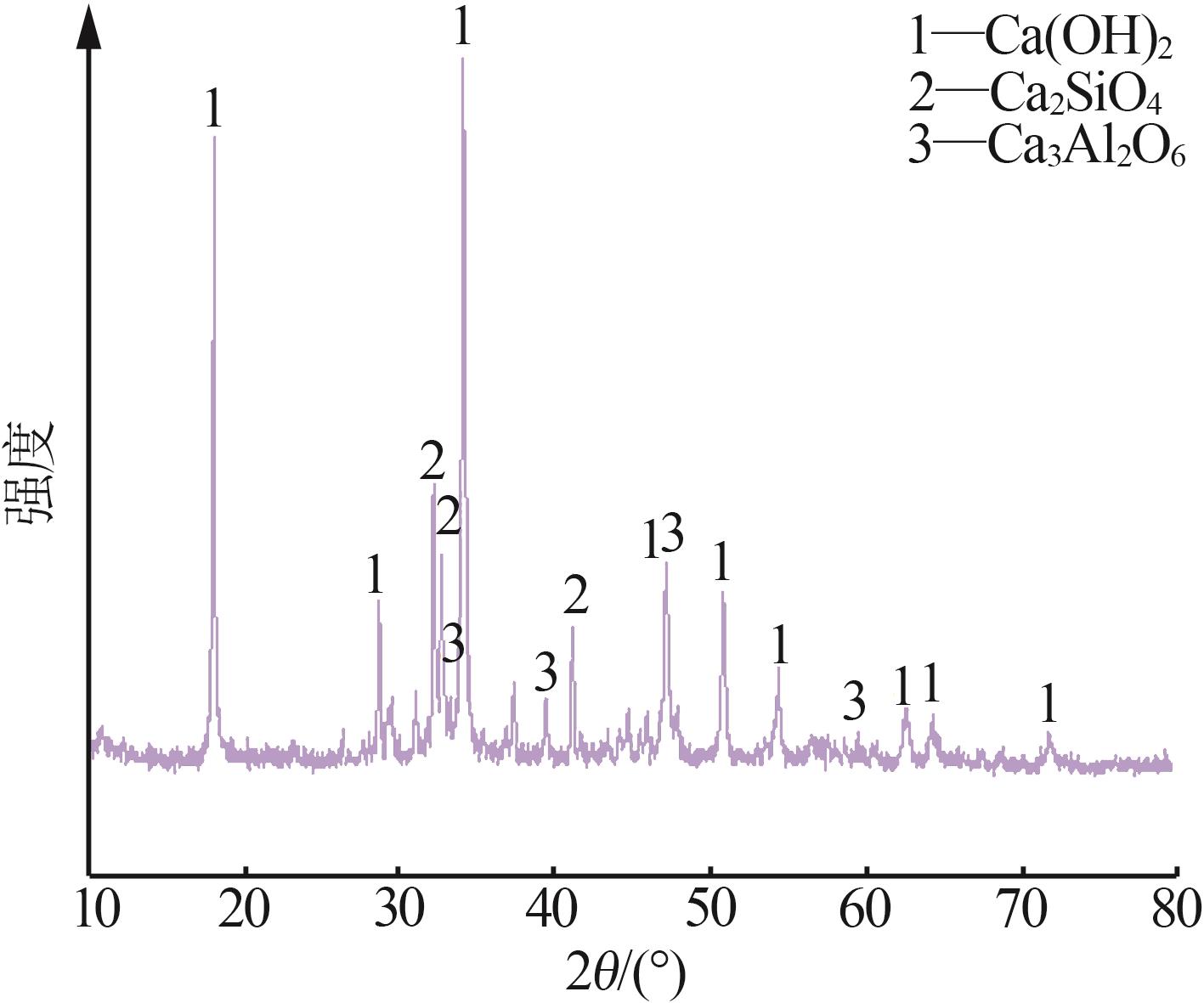
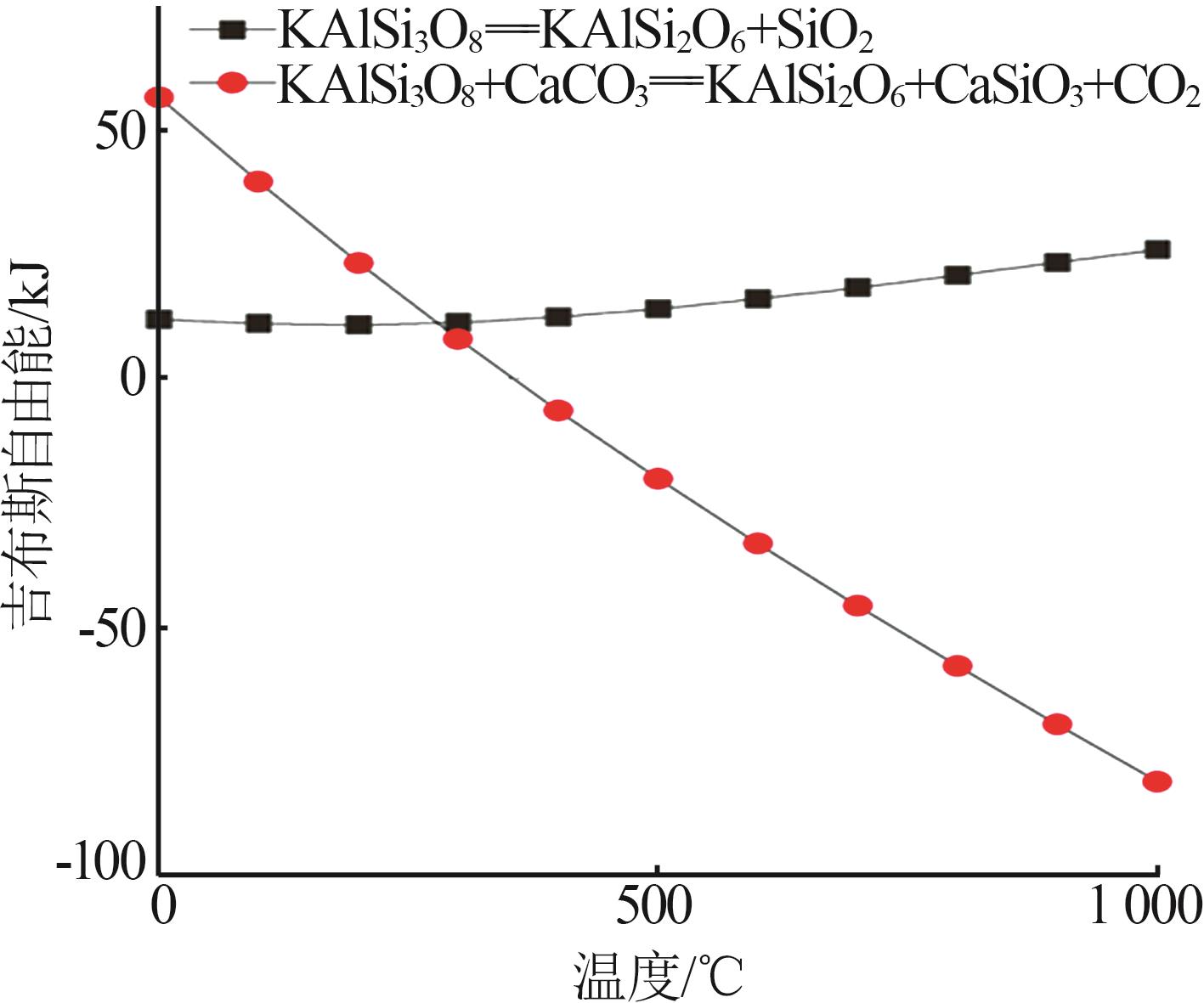
| 1 |
Survey U.S.Geological.Mineral commodity summaries 2023[R].Reston VA:Rock Products,2023.
|
| 2 |
中华人民共和国自然资源部.中国矿产资源报告[R].北京:中华人民共和国自然资源部,2022.
|
| 3 |
SAMANTRAY J, ANAND A, DASH B,et al.Silicate minerals-potential source of potash:A review[J].Minerals Engineering,2022,179:107463.
|
| 4 |
罗征,杨静,马鸿文,等.氟化物助剂分解钾长石制取硫酸钾研究评述[J].化工矿物与加工,2018,47(8):65-70.
|
|
LUO Zheng, YANG Jing, MA Hongwen,et al.Research review of preparation of potassium sulfate from K-feldspar with fluoride as additive agent[J].Industrial Minerals & Processing,2018,47(8):65-70.
|
| 5 |
WU Yusheng, LI Laishi, LIU Xiaofu,et al.Decomposition of K-feldspar by potassium hydroxide solution in the hydrothermal system[J].Minerals Engineering,2022,178:107392.
|
| 6 |
LIU Changjiang, MA Hongwen.Hydrothermal decomposition process of K-feldspar in NaOH solution[J].Asia-Pacific Journal of Chemical Engineering,2021,16(1):e2578.
|
| 7 |
XUE Xiaopeng, ZHANG Li, PENG Yunxiang,et al.Effects of mineral structure and microenvironment on K release from potassium aluminosilicate minerals by Cenococcum geophilum fr[J].Geomicrobiology Journal,2019,36(1):11-18.
|
| 8 |
XUE Xiaopeng, WANG Wei, FAN Hao,et al.Adsorption behavior of oxalic acid at water-feldspar interface:Experiments and molecular simulation[J].Adsorption,2019,25(6):1191-1204.
|
| 9 |
姜炜,罗孟杰,刘程琳,等.亚熔盐低温浸取钾长石工艺过程[J].华东理工大学学报(自然科学版),2019,45(2):206-215.
|
|
JIANG Wei, LUO Mengjie, LIU Chenglin,et al.Leaching process of potassium feldspar by sub-molten salt at low temperature[J].Journal of East China University of Science and Technology,2019,45(2):206-215.
|
| 10 |
董安瑞,罗孟杰,姜炜,等.亚熔盐分解钾长石矿样中钾铝硅的分离[J].过程工程学报,2018,18(5):972-980.
|
|
DONG Anrui, LUO Mengjie, JIANG Wei,et al.Separation and recovery of potassium,aluminum and silicon after decomposition of potassium feldspar using sub-molten salt method[J].The Chinese Journal of Process Engineering,2018,18(5):972-980.
|
| 11 |
SHANGGUAN Wenjie, SONG Jimin, YUE Hairong,et al.An efficient milling-assisted technology for K-feldspar processing,industrial waste treatment and CO2 mineralization[J].Chemical Engineering Journal,2016,292:255-263.
|
| 12 |
孟姣,赵建海,史欢欢.微波对钾长石在碱性条件下低温提钾性能的影响[J].无机盐工业,2017,49(12):20-22,52.
|
|
MENG Jiao, ZHAO Jianhai, SHI Huanhuan.Effect of microwave on potassium extraction performance in alkaline solution from potassium feldspar under low temperature[J].Inorganic Chemicals Industry,2017,49(12):20-22,52.
|
| 13 |
耿锐仙,夏举佩,杨劲,等.钾长石助熔磷矿石碳热反应热力学分析与评价[J].化学工程,2017,45(8):54-59.
|
|
GENG Ruixian, XIA Jupei, YANG Jin,et al.Thermodynamic analysis and evaluation of thermal reaction of phosphate ore with K-feldspar fluxing[J].Chemical Engineering(China),2017,45(8):54-59.
|
| 14 |
LU Dinghui, CHEN Qianlin, LI Cuiqin,et al.Effect of potassium feldspar on the decomposition rate of phosphogypsum[J].Journal of Chemical Technology and Biotechnology,2021,96(2):374-383.
|
| 15 |
YUAN Bo, LI Chun, LIANG Bin,et al.Extraction of potassium from K-feldspar via the CaCl2 calcination route[J].Chinese Journal of Chemical Engineering,2015,23(9):1557-1564.
|
| 16 |
SHI Yuling, JIANG Youfa, LIU Chenglin,et al.Effect of manganese salts on recovery of potassium from K-feldspar by means of a calcination reaction in the chloride salts-calcium carbonate system[J].Energy Sources,Part A:Recovery,Utilization,and Environmental Effects,2023,45(2):6150-6161.
|
| 17 |
ZHANG Yan, ASSELIN E, LI Zhibao.Laboratory and pilot scale studies of potassium extraction from K-feldspar decomposition with CaCl2 and CaCO3 [J].Journal of Chemical Engineering of Japan,2016,49(2):111-119.
|
| 18 |
WANG Chao, YUE Hairong, LI Chun,et al.Mineralization of CO2 using natural K-feldspar and industrial solid waste to produce soluble potassium[J].Industrial & Engineering Chemistry Research,2014,53(19):7971-7978.
|
| 19 |
ZHONG Yiwei, GAO Jintao, CHEN Panze,et al.Recovery of potassium from K-feldspar by thermal decomposition with flue gas desulfurization gypsum and CaCO3:Analysis of mechanism and kinetics[J].Energy & Fuels,2017,31(1):699-707.
|
| 20 |
汪碧容,石林.钾长石—硫酸钙—碳酸钙体系的热分解过程分析[J].化工矿物与加工,2011,40(3):12-15.
|
|
WANG Birong, SHI Lin.Study on thermal decomposition of potash feldspar-CaSO4-CaCO3 system[J].Industrial Minerals & Processing,2011,40(3):12-15.
|
| 21 |
石林,曾小平,柴妮,等.添加剂对KAlSi3O8-CaSO4-CaCO3体系反应表观活化能的影响[J].岩石矿物学杂志,2010,29(1):90-94.
|
|
SHI Lin, ZENG Xiaoping, CHAI Ni,et al.The effects of the addition of four types of fusing agents on the apparent activation energy of the KAlSi3O8-CaSO4-CaCO3 system[J].Acta Petrologica et Mineralogica,2010,29(1):90-94.
|
 ), KUANG Fei1, LIU Chenglin1,2(
), KUANG Fei1, LIU Chenglin1,2( )
)