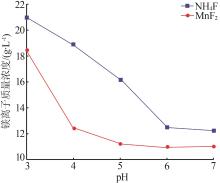
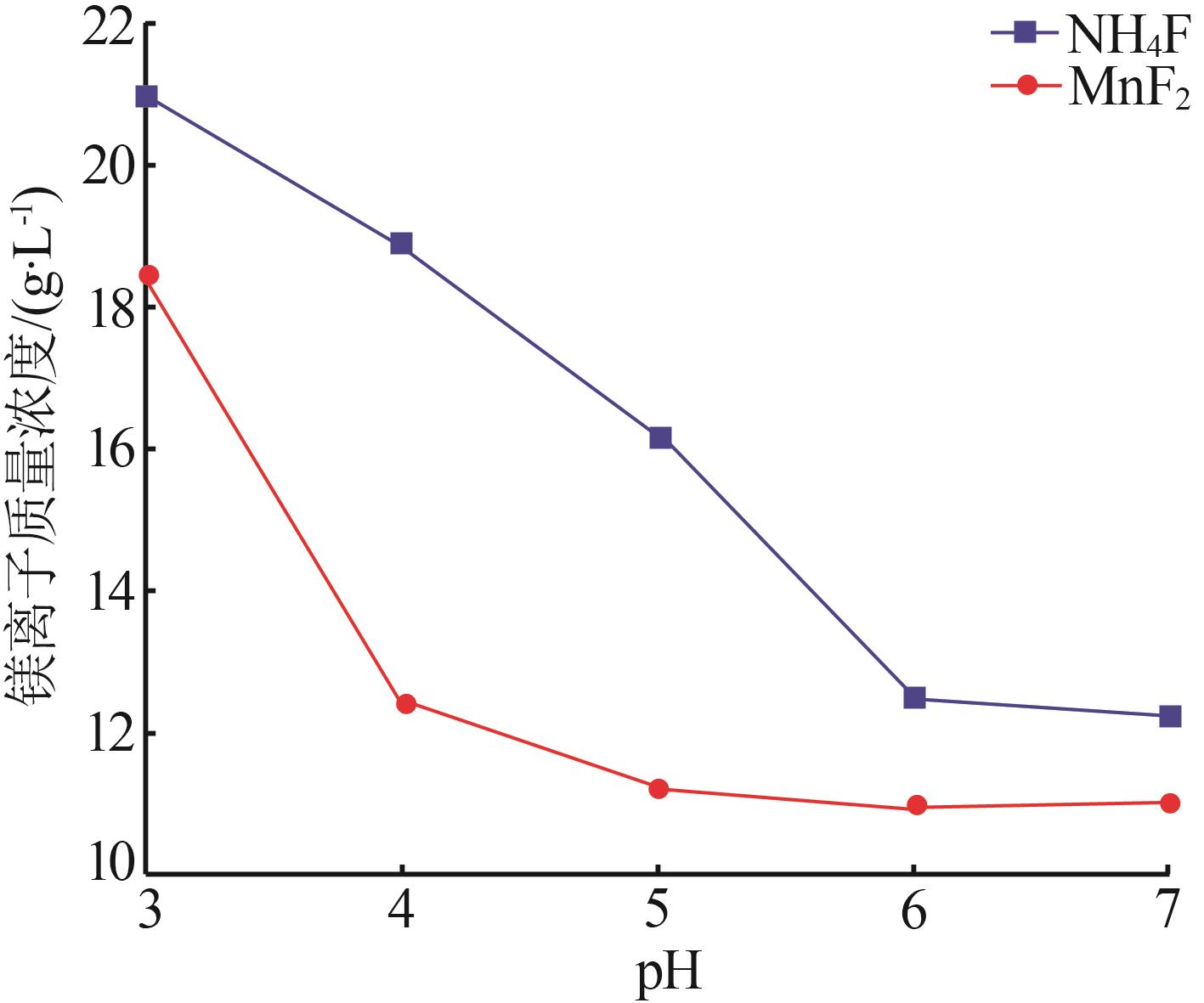
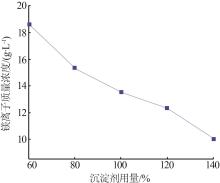
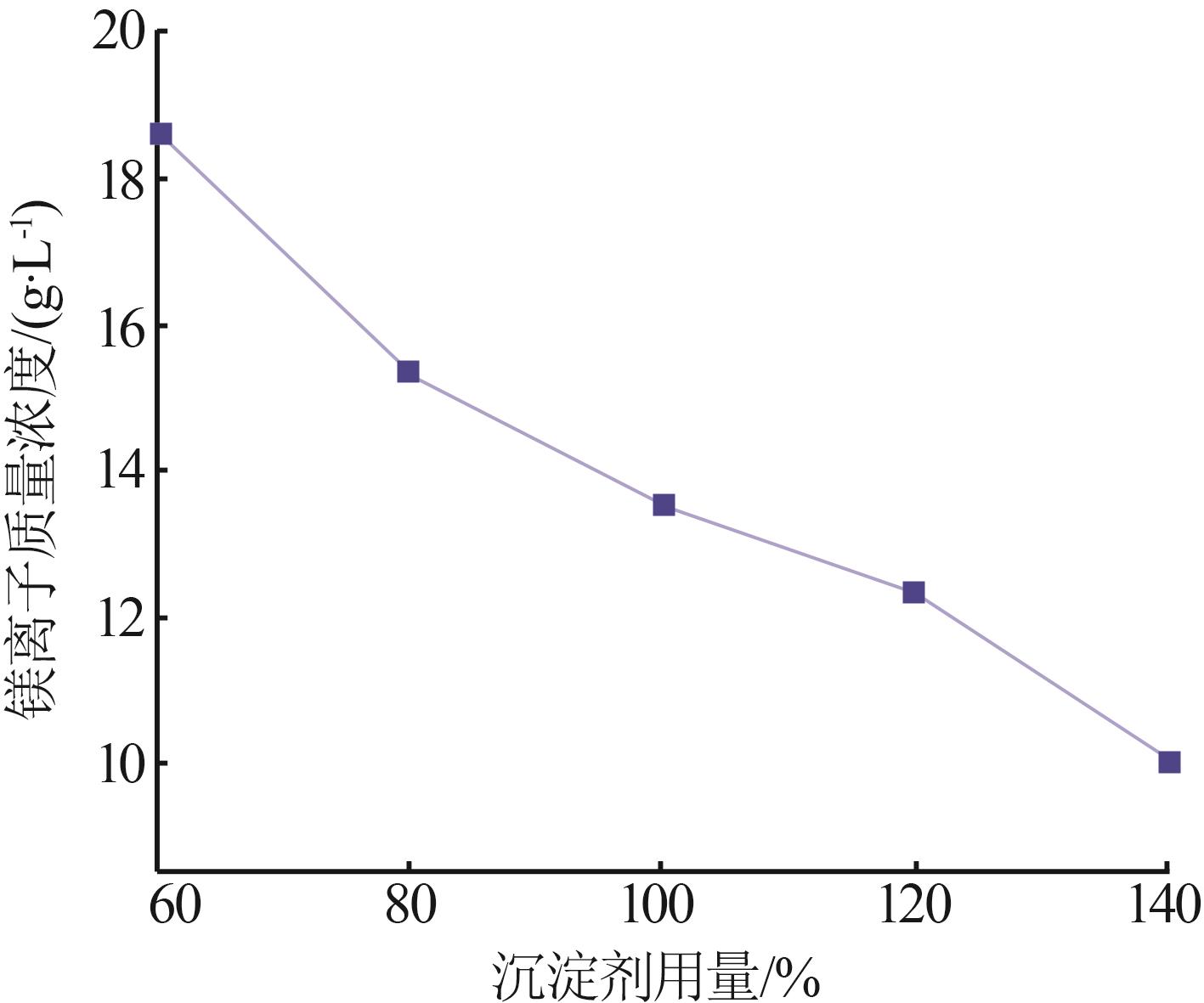
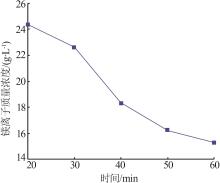
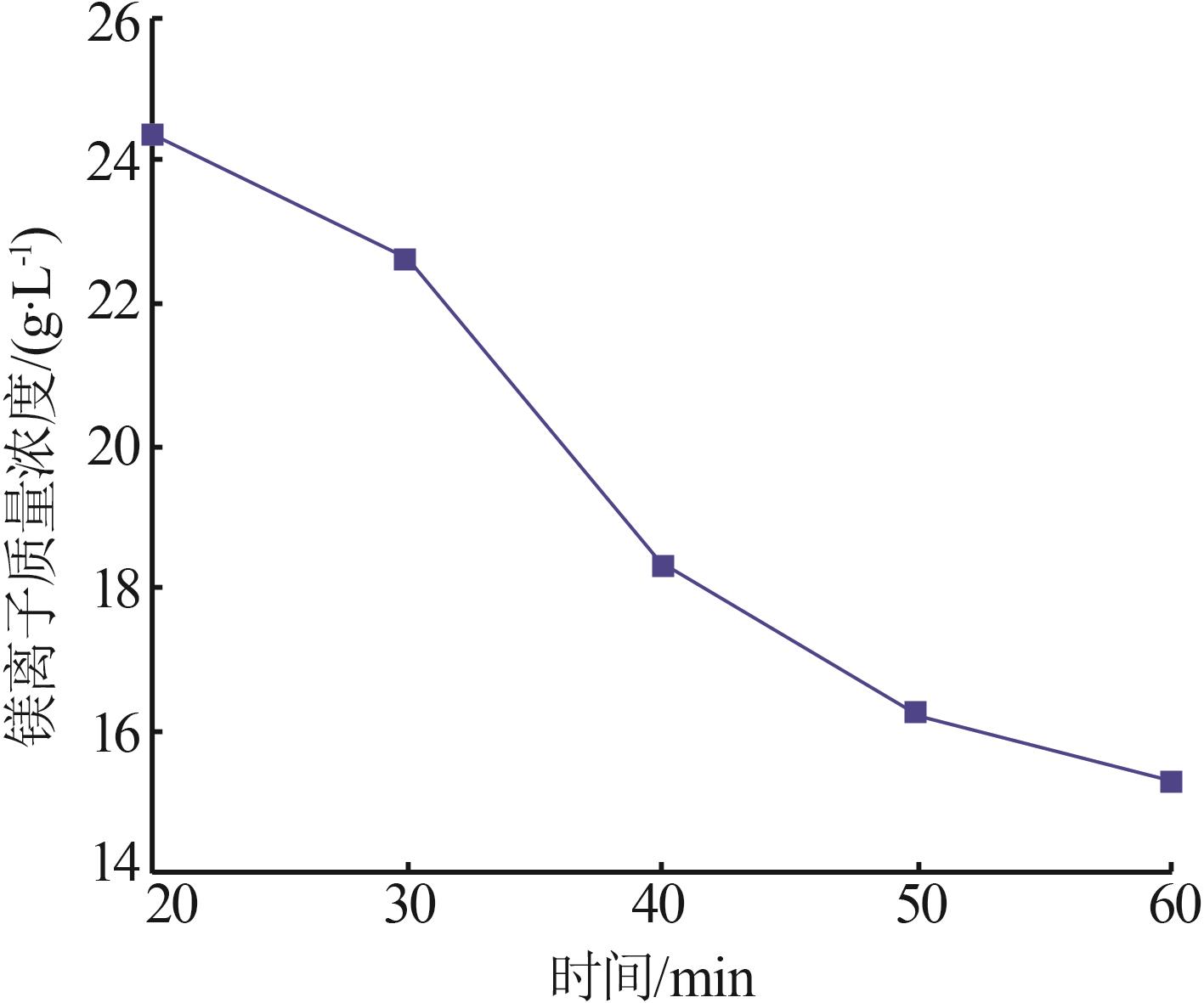
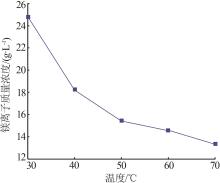
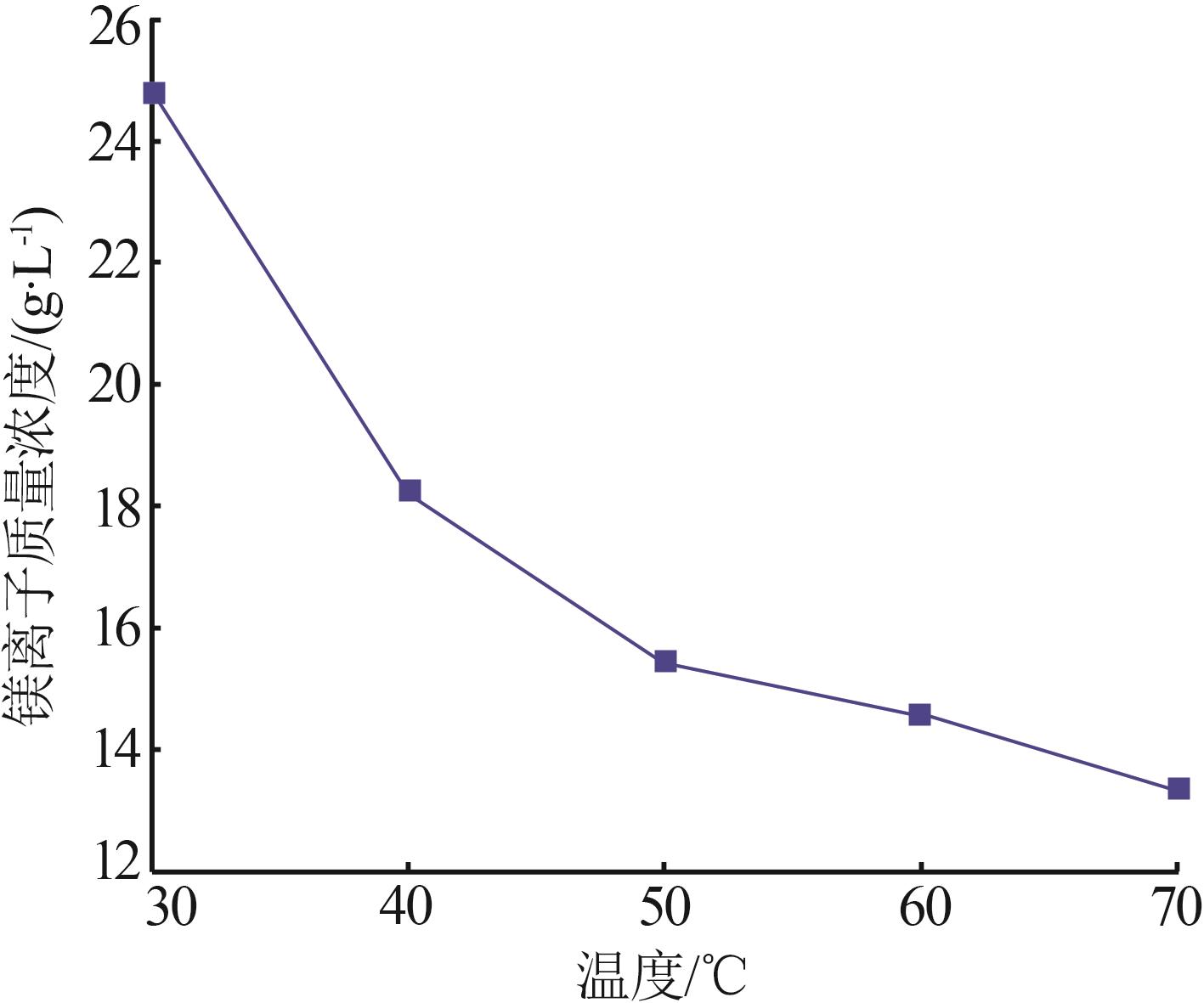
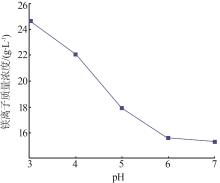
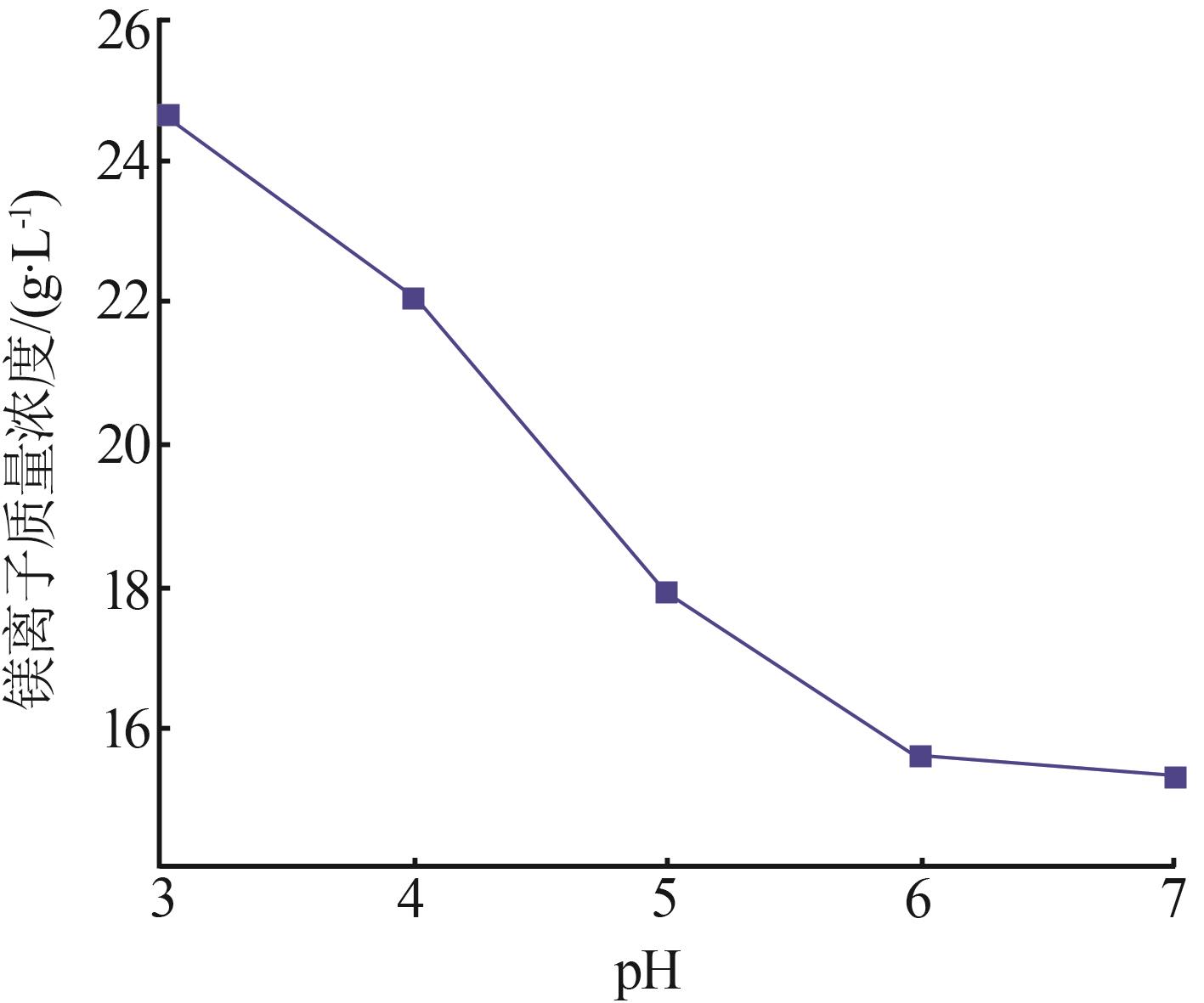
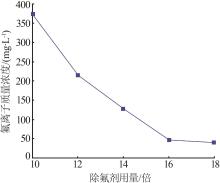
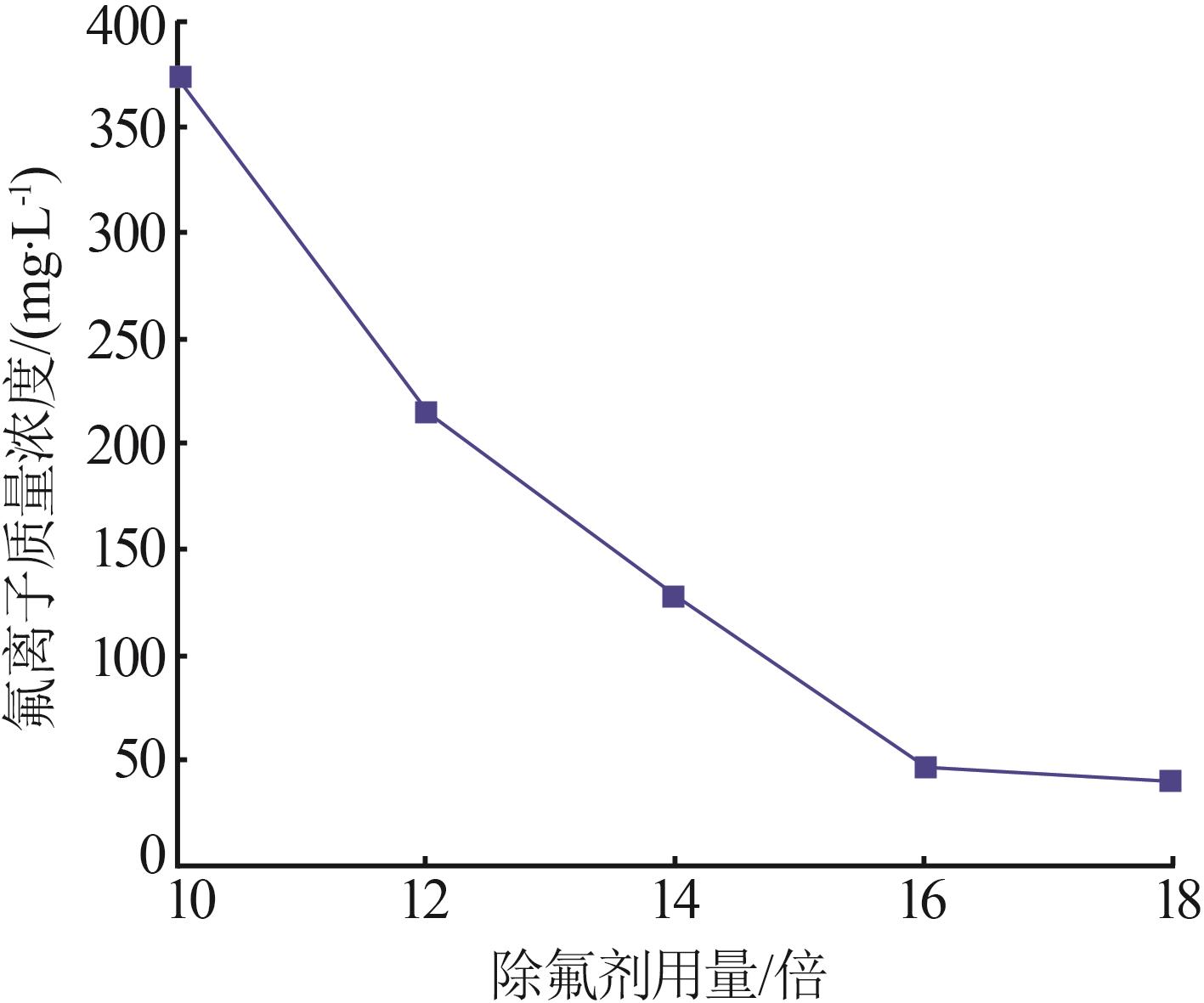
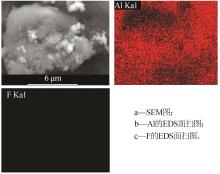
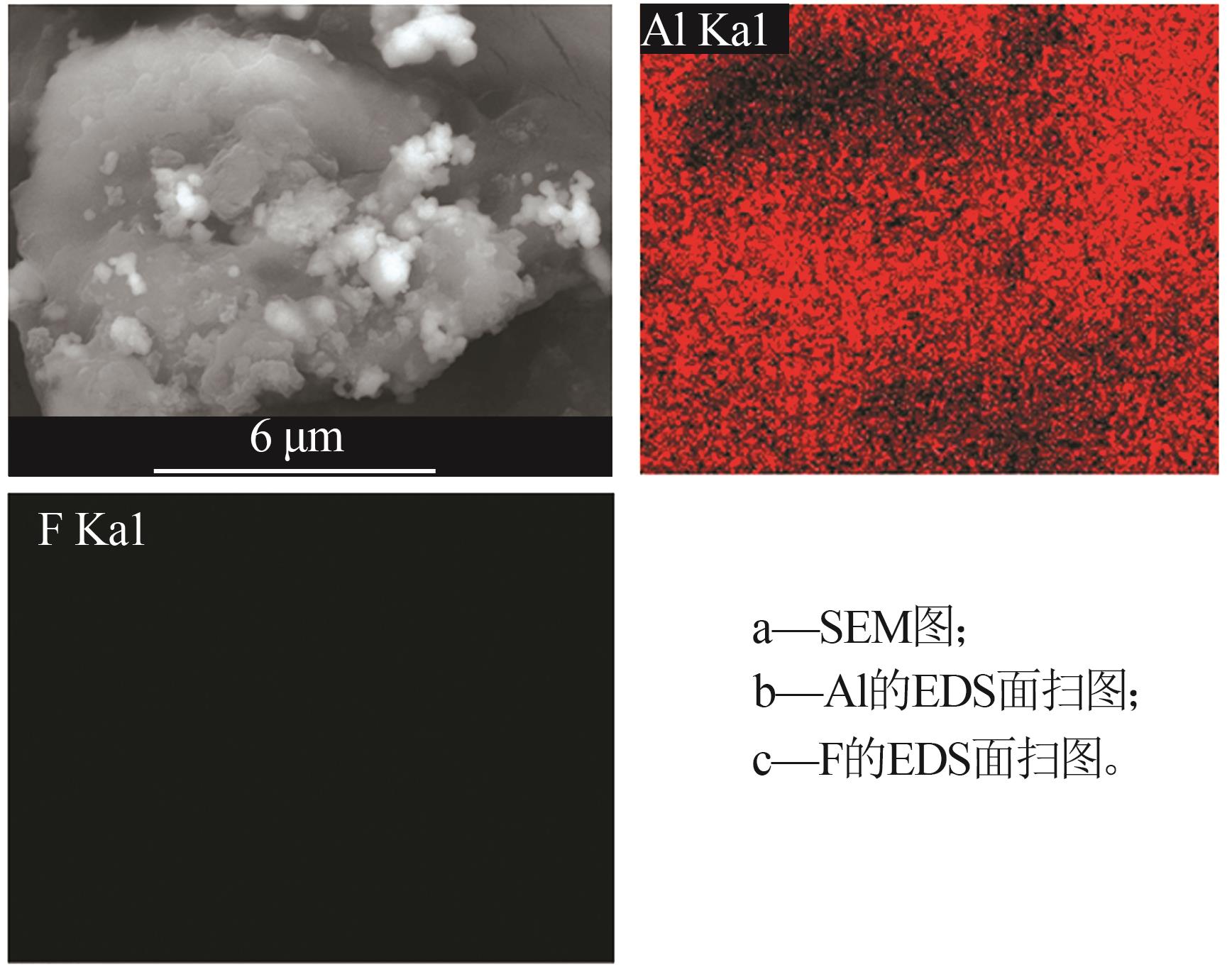
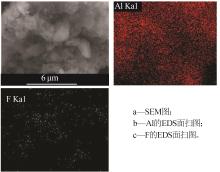
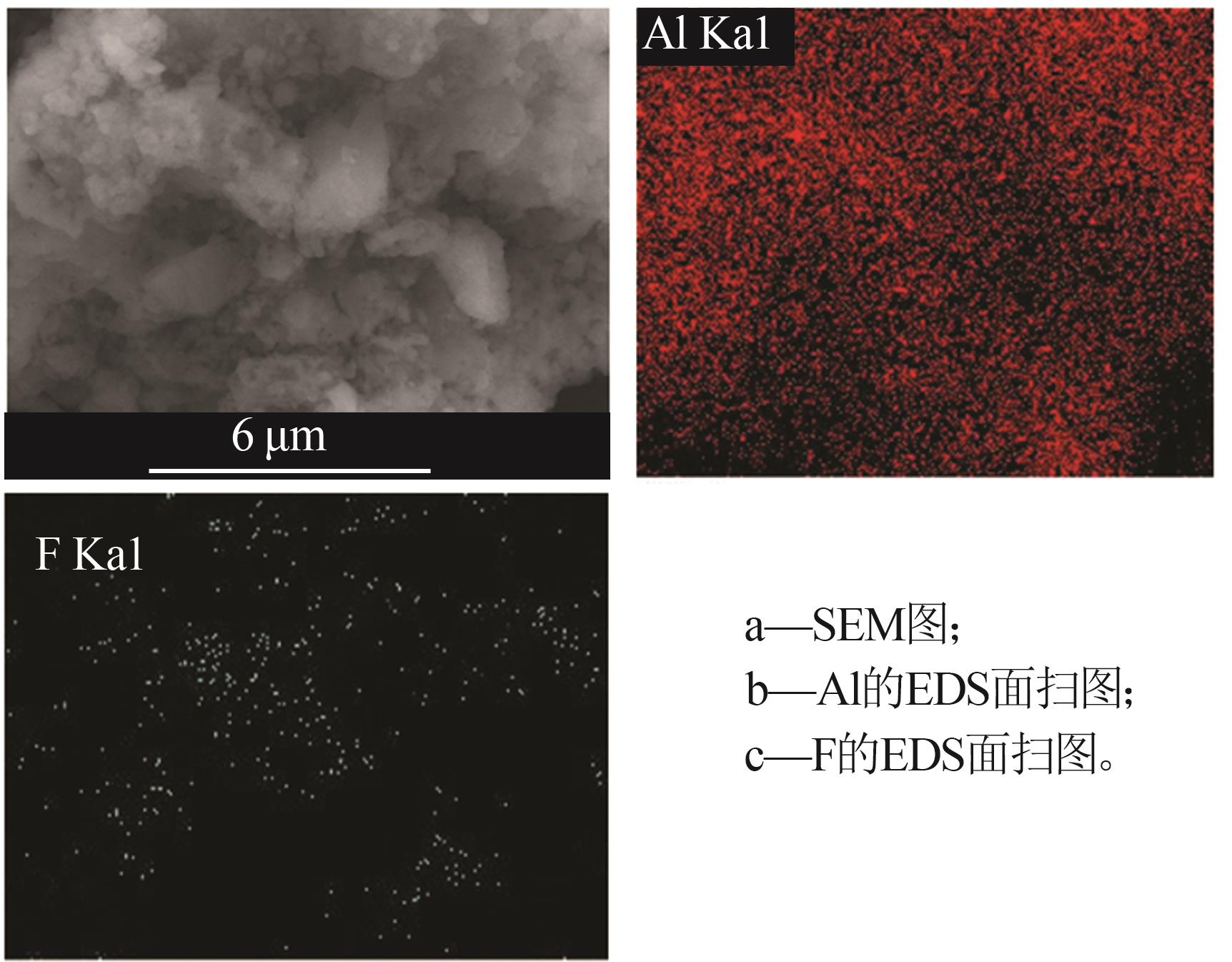
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (6): 40-45.doi: 10.19964/j.issn.1006-4990.2023-0454
• Research & Development • Previous Articles Next Articles
Study on purification of magnesium from manganese electrolyte by fluorine salt method
LU Ju1,2( ), WANG Jiawei1,2,3, WANG Haifeng1,2,3(
), WANG Jiawei1,2,3, WANG Haifeng1,2,3( ), PEI Zhengqing1,2, ZHOU Xingjie1,2, ZHENG Kexin1,2, MA Dehua1,2
), PEI Zhengqing1,2, ZHOU Xingjie1,2, ZHENG Kexin1,2, MA Dehua1,2
- 1.College of materials and metallurgy,Guizhou University,Guiyang 550025
2.Guizhou Key Laboratory of metallurgical engineering and process energy conservation,Guiyang 550025
3.Engineering Technology and Research Center of Manganese Material for Battery,Tongren 554300
-
Received:2023-09-11Online:2024-06-10Published:2024-06-20 -
Contact:WANG Haifeng E-mail:2642656692@qq.com;380889450@qq.com
CLC Number:
Cite this article
LU Ju, WANG Jiawei, WANG Haifeng, PEI Zhengqing, ZHOU Xingjie, ZHENG Kexin, MA Dehua. Study on purification of magnesium from manganese electrolyte by fluorine salt method[J]. Inorganic Chemicals Industry, 2024, 56(6): 40-45.
share this article
| 1 | 宋瀚文,张辉,宋达,等.硫酸锰制备工艺研究进展[J].盐科学与化工,2023,52(2):10-12. |
| SONG Hanwen, ZHANG Hui, SONG Da,et al.Research progress on preparation technology of manganese sulfate[J].Journal of Salt Science and Chemical Industry,2023,52(2):10-12. | |
| 2 | ZHANG Wensheng, CHENG Chuyong.Manganese metallurgy review.Part I:Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide[J].Hydrometallurgy,2007,89(3/4):137-159. |
| 3 | MENDONÇA DE ARAUJO J A, REIS DE CASTRO M M,DE FREITAS CUNHA LINS V.Reuse of furnace fines of ferro alloy in the electrolytic manganese production[J].Hydrometallurgy,2006,84(3/4):204-210. |
| 4 | 王晓东,张衡,彭碧君.钙法提钒废水除锰并制备高纯碳酸 锰[J].化工环保,2022,42(3):293-297. |
| WANG Xiaodong, ZHANG Heng, PENG Bijun.Removal of manganese from vanadium extraction wastewater by calcium process and preparation of high-purity manganese carbonate[J].Environmental Protection of Chemical Industry,2022,42(3):293-297. | |
| 5 | TANG Liang, HE Zhaoyi, CHEN Kefan,et al.Study of microscopic properties and heavy metal solidification mechanism of electrolytic manganese residue-based cementitious materials[J].Environmental Science and Pollution Research,2023,30(48):105056-105071. |
| 6 | 李重洋,钱振,时启龙,等.含镁三元系锰电解液理化性能研究[J].矿冶工程,2019,39(4):79-82,88. |
| LI Chongyang, QIAN Zhen, SHI Qilong,et al.Physical-chemical properties of ternary Mg-containing manganese electrolyte[J].Mining and Metallurgical Engineering,2019,39(4):79-82,88. | |
| 7 | 郑子恩.电解金属锰阳极泥还原焙烧-酸浸试验研究[J].稀有金属与硬质合金,2022,50(2):15-18. |
| ZHENG Zien.Experimental research on reduction roasting-acid leaching of electrolytic manganese anode slime[J].Rare Metals and Cemented Carbides,2022,50(2):15-18. | |
| 8 | 钱振,谭杰,李重洋,等.电解锰工业生产过程中镁组分迁移行为研究[J].矿冶工程,2022,42(4):112-115. |
| QIAN Zhen, TAN Jie, LI Chongyang,et al.Migration behavior of magnesium components in industrial production of electrolytic manganese[J].Mining and Metallurgical Engineering,2022,42(4):112-115. | |
| 9 | SHANG Yabo, WANG Yadong, LI Keqian,et al.Nucleation crystallization pelleting process for highly efficient manganese ion recovery in electrolytic manganese wastewater[J].Chemical Engineering Journal,2023,475:146271. |
| 10 | 耿叶静,刘静,周娥,等.富镁软锰矿中镁的预脱除实验研究[J].中国锰业,2012,30(2):29-31,38. |
| GENG Yejing, LIU Jing, ZHOU E,et al.Research on pre-removal of Mg in rich Mg pyrolusite[J].China′s Manganese Industry,2012,30(2):29-31,38. | |
| 11 | 蔡振勇,易清风,刘汉勇,等.废铁屑还原软锰矿制备高纯硫酸锰工艺研究[J].中国锰业,2011,29(3):28-31. |
| CAI Zhenyong, YI Qingfeng, LIU Hanyong,et al.Preparation of high purity manganese sulfate from the reduction of pyrolusite using scrap iron as the reductant[J].China’s Manganese Industry,2011,29(3):28-31. | |
| 12 | 蒋文杰,张昭.硫酸锰溶液中镁离子的沉淀行为研究[J].无机盐工业,2014,46(10):34-38. |
| JIANG Wenjie, ZHANG Zhao.Precipitation behavior of magnesium ion in manganese sulfate solution[J].Inorganic Chemicals Industry,2014,46(10):34-38. | |
| 13 | 尤晓宇,王家伟,王海峰,等.电解锰生产用硫酸锰、镁、铵复盐体系结晶的研究[J].应用化工,2021,50(9):2353-2356. |
| YOU Xiaoyu, WANG Jiawei, WANG Haifeng,et al.Study on crystallization of manganese sulfate,magnesium sulfate and ammonium sulfate for electrolytic manganese production[J].Applied Chemical Industry,2021,50(9):2353-2356. | |
| 14 | 杨绍泽,任博,王春侠.电解金属锰加工工艺中乙醇循环除镁工艺初探[J].中国锰业,2011,29(2):15-17,24. |
| YANG Shaoze, REN Bo, WANG Chunxia.The study of magnesium removing by ethanol recycling for the technology of electrolytic manganese processing[J].China′s Manganese Industry,2011,29(2):15-17,24. | |
| 15 | 李萌,朱彤,张翔宇,等.纳滤膜处理含锰废水[J].化工环保,2012,32(3):260-263. |
| LI Meng, ZHU Tong, ZHANG Xiangyu,et al.Treatment of man-ganese-containing wastewater using nanofiltration membrane[J].Environmental Protection of Chemical Industry,2012,32(3):260-263. | |
| 16 | 谢子楠,王蛟,沈家国.工业硫酸锰中钙、镁的净化研究[J].无机盐工业,2015,47(5):48-50. |
| XIE Zinan, WANG Jiao, SHEN Jiaguo.Research on purification of Ca(Ⅱ) and Mg(Ⅱ) in industrial manganese sulfate[J].Inorganic Chemicals Industry,2015,47(5):48-50. | |
| 17 | GUIMARÃES A S, MANSUR M B.Solvent extraction of calcium and magnesium from concentrate nickel sulfate solutions using D2HEPA and Cyanex 272 extractants[J].Hydrometallurgy,2017,173:91-97. |
| 18 | ZHOU Libo, CHEN Ping, HU Cheng,et al.Study on the mechanical properties and hydration behavior of steel slag-red mud-electrolytic manganese residue based composite mortar[J].Applied Sciences,2023,13(10):5913. |
| 19 | 张彭汝,王文磊,杨超,等.硫酸锰溶液氟化沉淀法除镁的研究[J].有色金属(冶炼部分),2012(12):1-4. |
| ZHANG Pengru, WANG Wenlei, YANG Chao,et al.Study on removal of magnesium from manganese sulfate solution with fluorination precipitation[J].Nonferrous Metals(Extractive Metallurgy),2012(12):1-4. | |
| 20 | 刘洪刚,朱国才.氟化锰沉淀脱除还原氧化锰矿浸出液中钙镁[J].矿冶,2007,16(4):25-28,65. |
| LIU Honggang, ZHU Guocai.Removal of Ca(Ⅱ),Mg(Ⅱ) from leaching solution of low-grade manganese ore by precipitation with fluoride[J].Mining and Metallurgy,2007,16(4):25-28,65. | |
| 21 | 秦吉涛.Mg2+在锰电解体系中的分布及其行为研究[D].贵阳:贵州大学,2019. |
| QIN Jitao.Research on distribution and behavior of Mg2+ in manganese electrolysis system[D].Guiyang:Guizhou University,2019. | |
| 22 | 秦吉涛,王家伟,王海峰,等.Mg2+对硫酸锰电解液理化性质的影响[J].有色金属(冶炼部分),2019(1):12-15,30. |
| QIN Jitao, WANG Jiawei, WANG Haifeng,et al.Effect of Mg2+ on physicochemical properties of manganese sulfate electrolyte[J].Nonferrous Metals(Extractive Metallurgy),2019(1):12-15,30. | |
| 23 | 王海峰,王家伟.一种纳米除氟剂及其制备方法和应用:中国,116786097A[P].2023-09-22. |
| 24 | 时海平,王东田,田美玲.活性氧化铝的制备及除氟性能研究[J].苏州科技学院学报(工程技术版),2010,23(3):23-26. |
| SHI Haiping, WANG Dongtian, TIAN Meiling.A research on preparation of activated alumina and its property of fluriode[J].Journal of Suzhou University of Science and Technology(Engineering and Technology),2010,23(3):23-26. |
| [1] | ZHENG Kexin, WANG Haifeng, WANG Jiawei, ZHOU Xingjie, PEI Zhengqing, LU Ju, MA Dehua. Study on leaching and kinetics of rhododengite in Jingxi area of Guangxi [J]. Inorganic Chemicals Industry, 2025, 57(1): 51-57. |
| [2] | SU Hang, SONG Jitian, HUANG Zhiqiang, DONG Qing, ZHANG Yaxiong. Study on crystallization kinetics of manganese sulfate monohydrate in H2SO4-H2O binary system [J]. Inorganic Chemicals Industry, 2024, 56(8): 40-46. |
| [3] | XIE Zi-Nan, WANG Jiao, SHEN Jia-Guo. Research on purification of Ca(Ⅱ) and Mg(Ⅱ) in industrial manganese sulfate [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(5): 48-. |
| [4] | HE Jia, ZHANG Zhao. Synthesis of Mn3O4 powder from manganese sulfate by precipitation-oxidation process [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(3): 45-. |
| [5] | LIN Qing-Quan, LIU You-Cai, LI Li-Feng, WANG Yun-Qiu, WANG Jia-Yi, XU Jing-Yan, XI Han-Xia, PENG Bin-Jiang, ZHU Zhong-Si. Study on technology of preparing high purity manganese sulfate with low-grade manganese carbonate ores [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(11): 35-. |
| [6] | JIANG Wen-Jie, ZHANG Zhao. Precipitation behavior of magnesium ion in manganese sulfate solution [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(10): 34-. |
| [7] | XIE Yu-Qi, 欧Yang-Hui-Xiang , HU Bo-Qi, LING Shao-Ming. Study on production process for high-purity manganese sulfate with waste sulphuric acid of titanium white-pyrolusite-pyrite [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(7): 49-. |
| [8] | BAO Xin-Jun, WANG Zhi-Jian, LIU Ji-Bo, SU Zheng-Fu, HUANG Zhi, CHENG Wei, ZHOU De-Bi. Purification of industrial manganese sulfate and preparation of high purity LiMn2O4 [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(5): 59-. |
| [9] | MA Yu-Wen, FENG Ya-Li, LI Hao-Ran. Preparation of Mn3O4 from manganese sulfate by solid-state ball milling reaction at room temperature [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(2): 17-. |
| [10] | CUI Yi-Shun. Study on sulfuric acid leaching process of manganese from low-grade pyrolusite with bagasse as reducing agent [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(10): 45-. |
| [11] | Huang Qimao;Wang Chunping;Xu Wangsheng;Pan Zhiquan. Research on technology of reductive leaching manganese sulfate from low-grade pyrolusite by sawdust [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(2): 0-0. |
| [12] | LI Xuan-Hai, JI Dan-Dan, SU Hai-Feng, PAN Liu-Ping, LI Jing, LI Jian, WEN Yan-Xuan. Study on production of manganese sulphate by high-temperature crystallizing process [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(12): 16-. |
| [13] | Deng Chunyuan;Liu Changfeng. Determination of arsenic in feed-grade manganese sulfate by hydrogen aresenide generation followed by arsenic antimony molybdenum blue spectrophotometry [J]. INORGANICCHEMICALSINDUSTRY, 2009, 0(12): 0-0. |
| [14] | Xia Wentang;Zhao Zhongwei;Ren Zhengde. Study on deep removal of molybdenum from manganese sulfate solution [J]. INORGANICCHEMICALSINDUSTRY, 2008, 0(6): 0-0. |
| [15] | Tian Zongping;Li Li;Zhu Jiezhong;Peng Shunlian. Technological study and industrial practice for high efficiency and energy saving production of manganese sulfate [J]. INORGANICCHEMICALSINDUSTRY, 2008, 0(12): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||