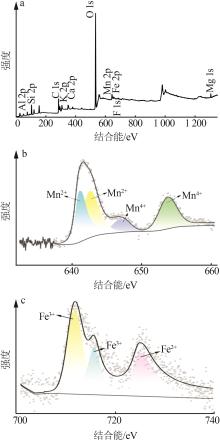
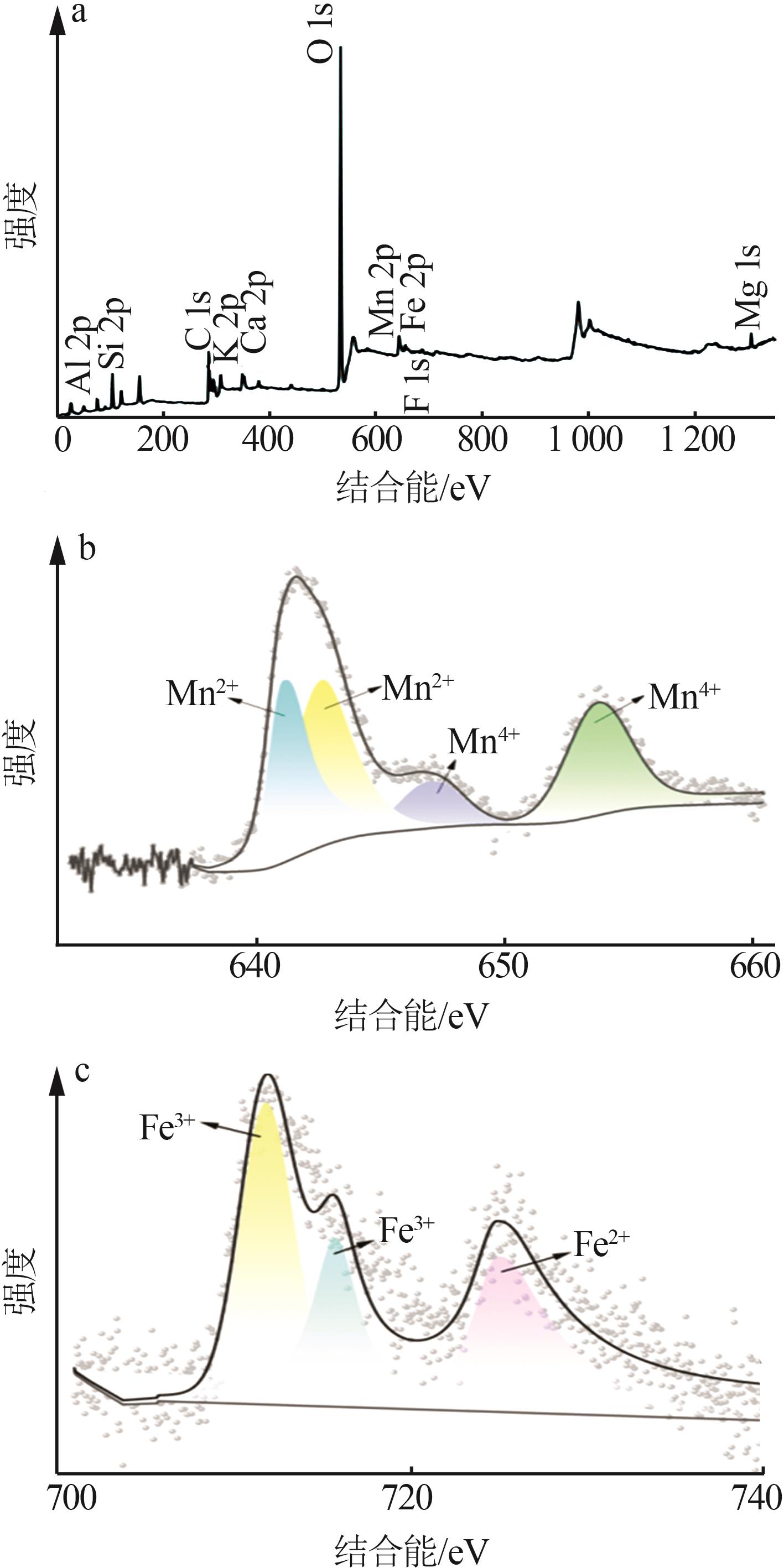
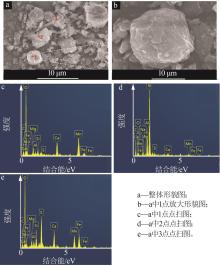
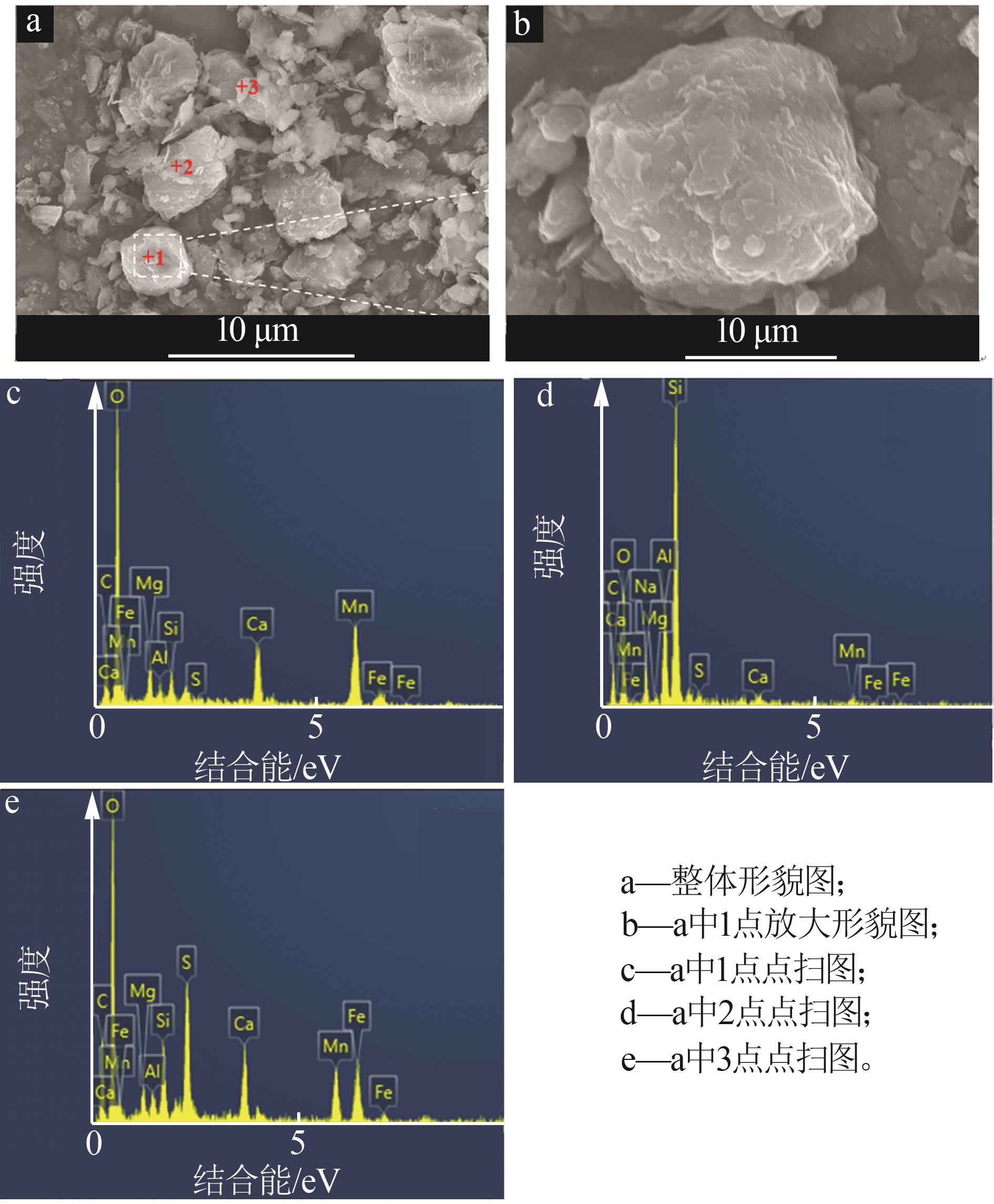
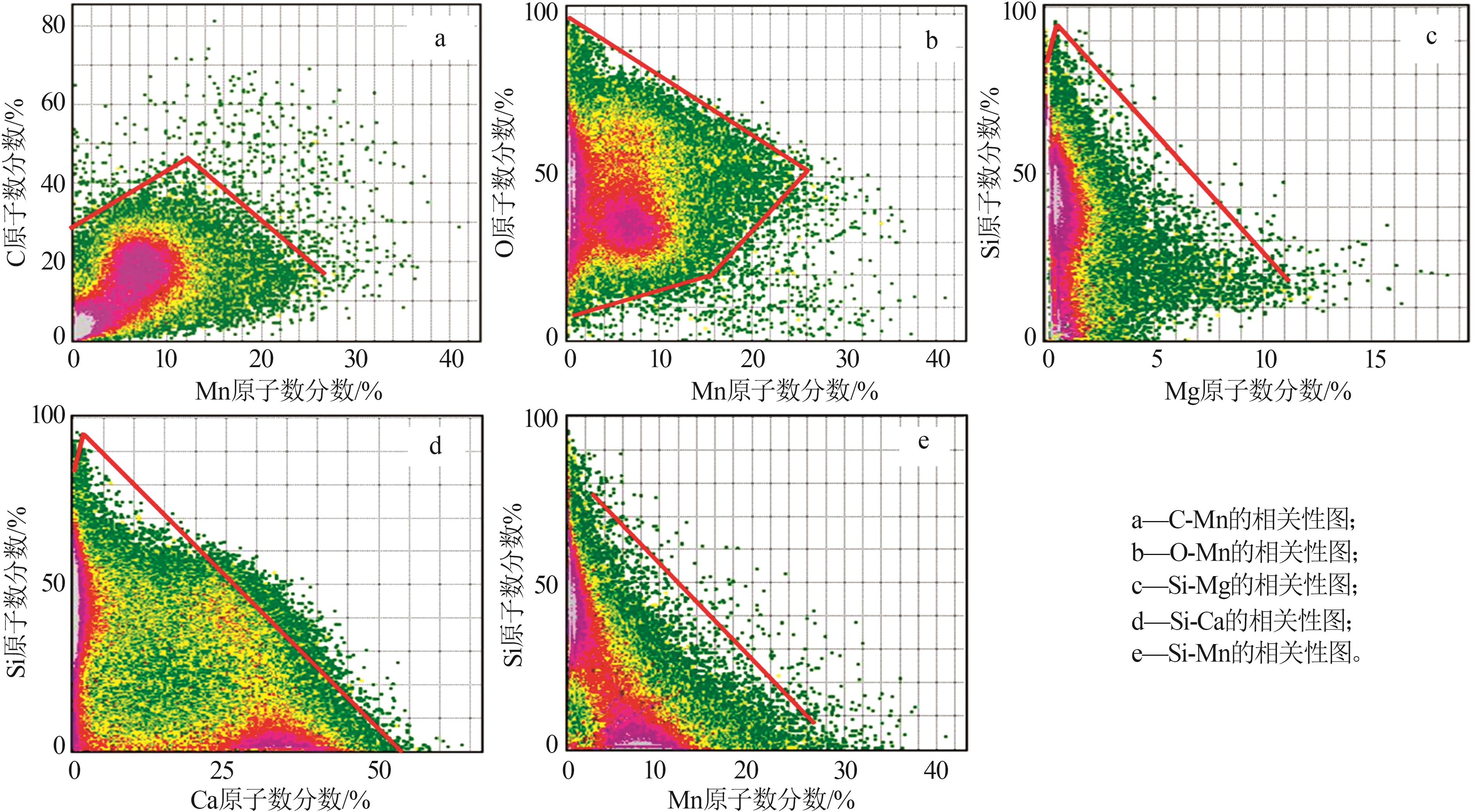
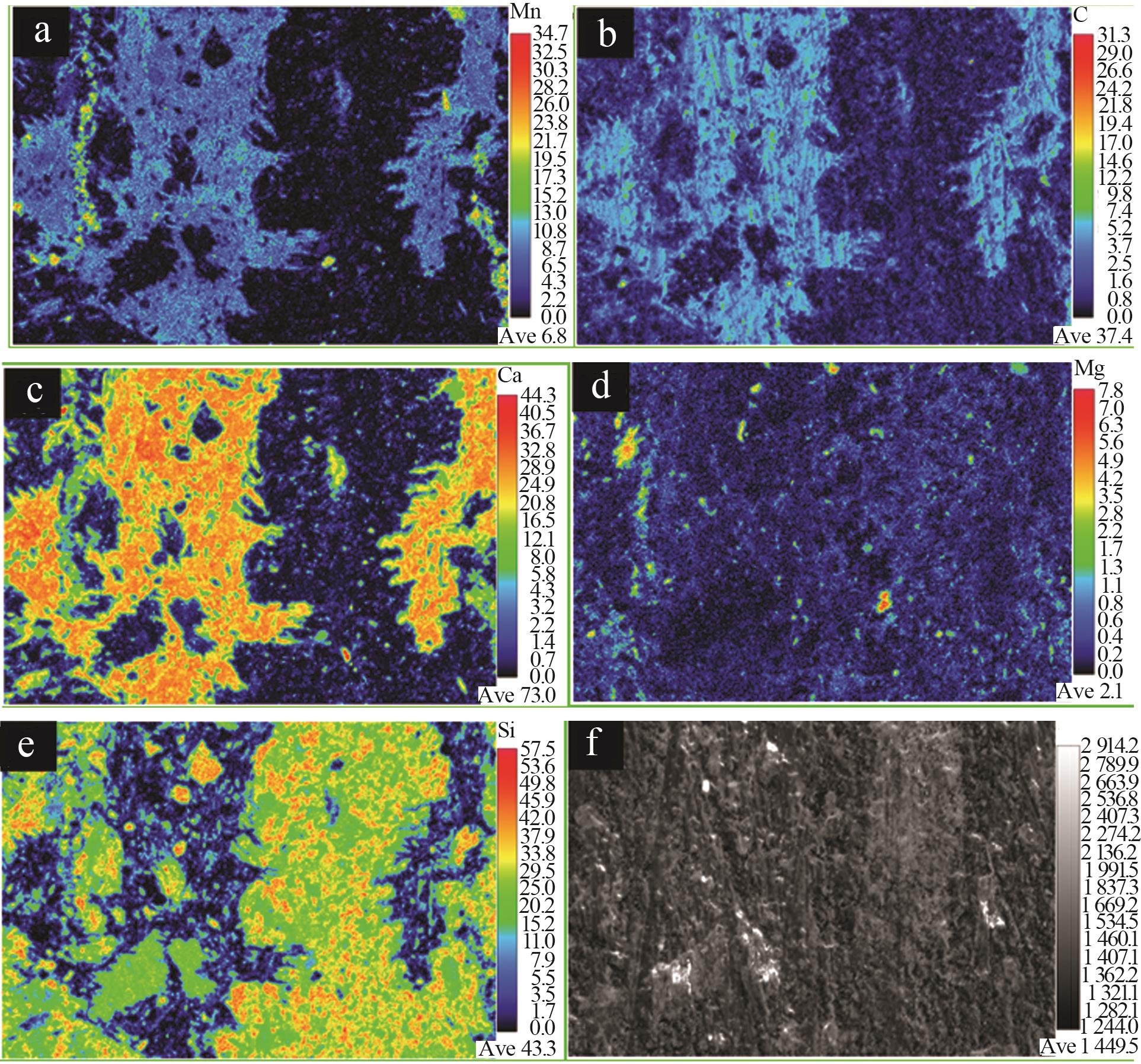
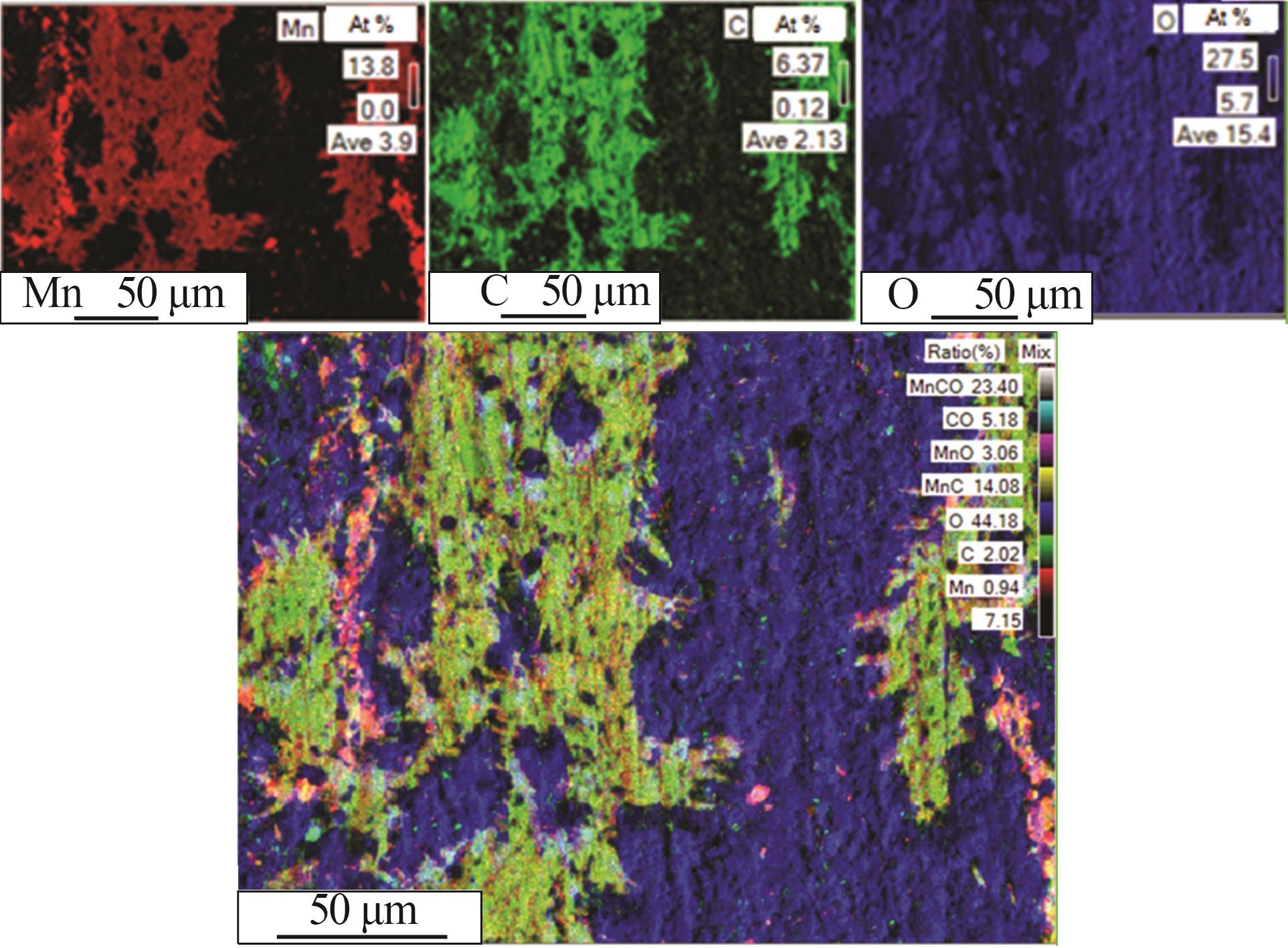
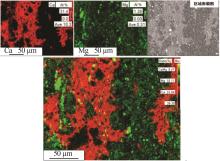
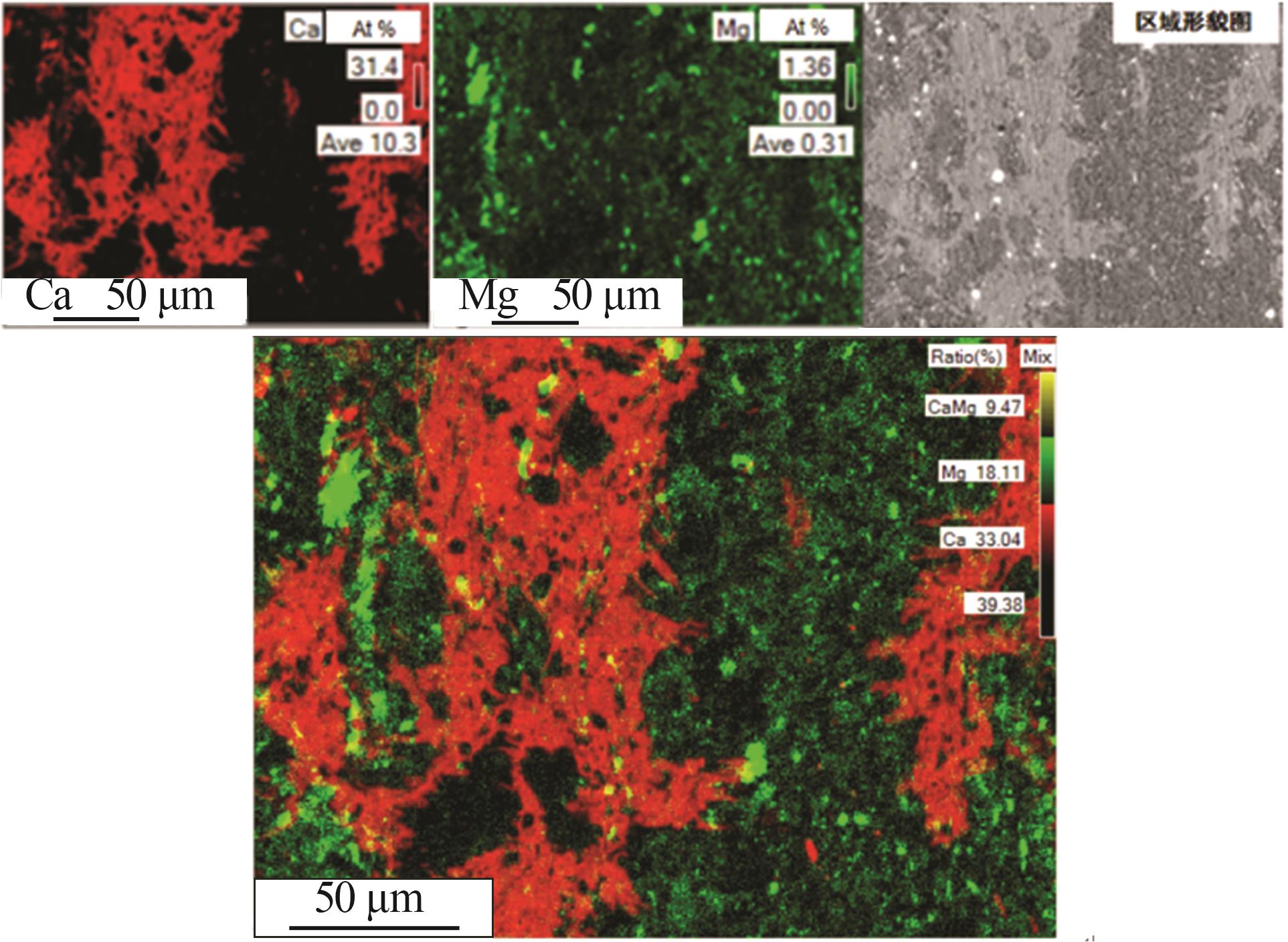
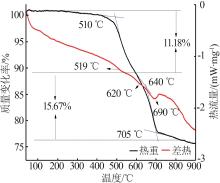
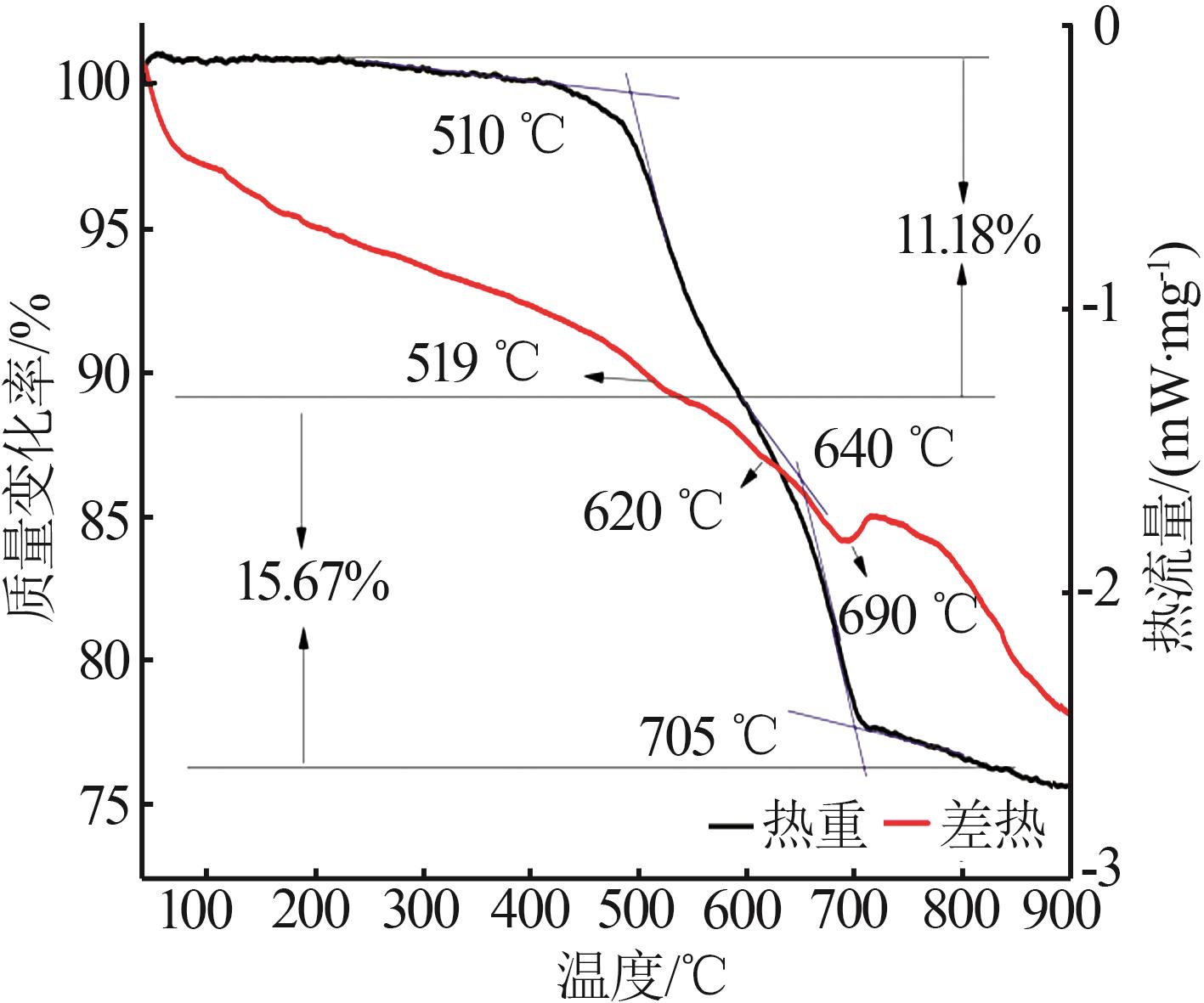
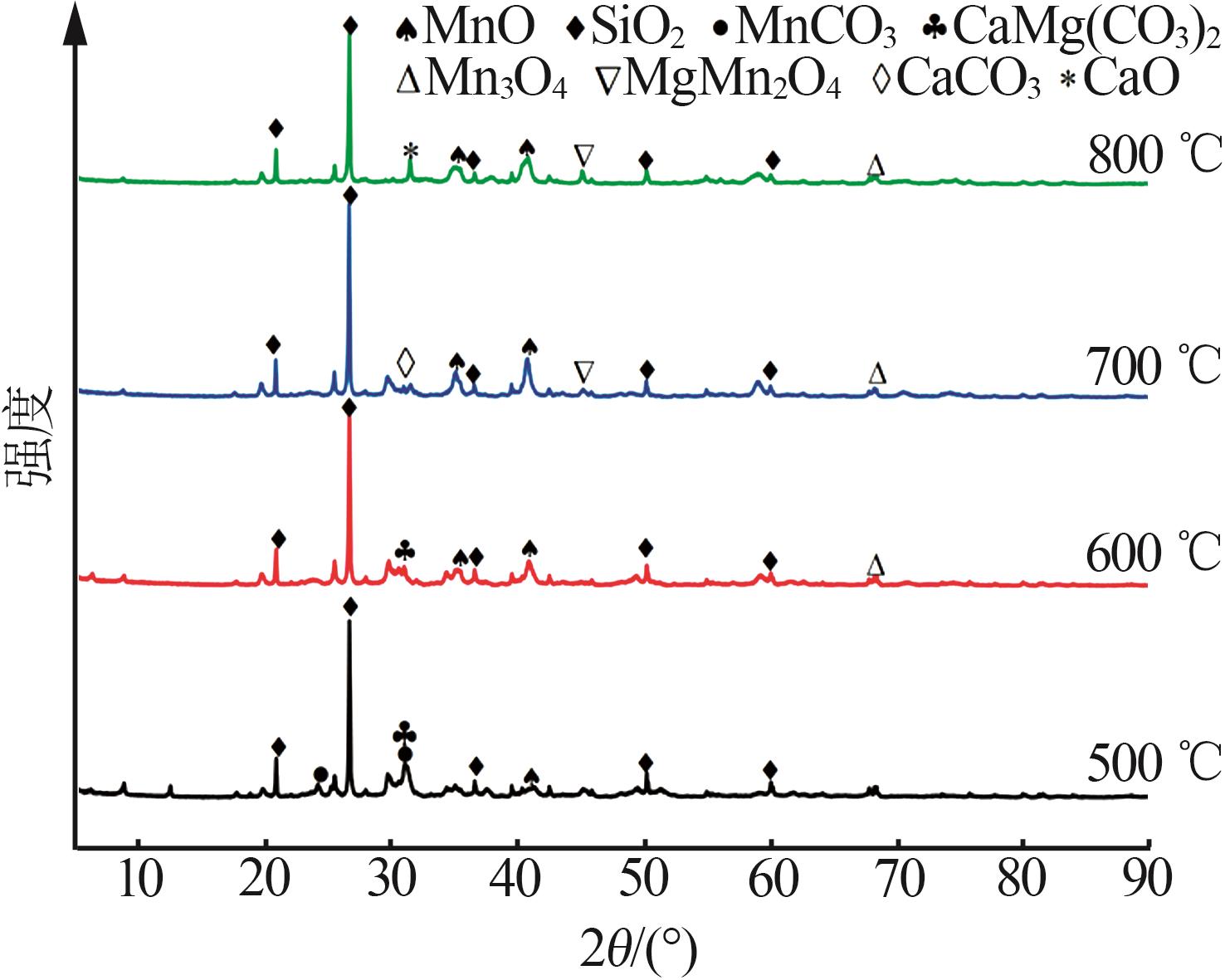
| 1 |
文馨蓓.浅谈中国锰矿资源的现状与潜力[J].西部资源,2015(5):142-143.
|
|
WEN Xinbei.Present situation and potential of manganese resources in China[J].Western Resources,2015(5):142-143.
|
| 2 |
任辉,刘敏,王自国,等.我国锰矿资源及产业链安全保障问题研究[J].中国工程科学,2022,24(3):20-28.
|
|
REN Hui, LIU Min, WANG Ziguo,et al.Security of manganese resources and industrial chain in China[J].Strategic Study of CAE,2022,24(3):20-28.
|
| 3 |
王彦,李艳军.某菱锰矿石工艺矿物学研究[J].金属矿山,2019(2):188-191.
|
|
WANG Yan, LI Yanjun.Research on process mineralogy of a carbonaceous rhodochrosite ore[J].Metal Mine,2019(2):188-191.
|
| 4 |
程湘,胡鹏,张海坤,等.锰矿主要类型、分布特点及开发现状[J].中国地质,2021,48(1):102-119.
|
|
CHENG Xiang, HU Peng, ZHANG Haikun,et al.The main types,distribution and current development of manganese ore deposi-ts[J].Geology in China,2021,48(1):102-119.
|
| 5 |
高艺,刘宏杰.锰矿资源现状及潜力预测[J].中国锰业,2020,38(2):1-5.
|
|
GAO Yi, LIU Hongjie.A current situation of manganese resources and its technical research progress[J].China′s Manganese Industry,2020,38(2):1-5.
|
| 6 |
张兴然.低品位复杂锰矿浸出与电解过程的强化研究[D].重庆:重庆大学,2017.
|
|
ZHANG Xingran.Research on intensification of leaching and electrolysis process for low grade and complex manganese ore[D].Chongqing:Chongqing University,2017.
|
| 7 |
WANG Kui, ZHANG Qiwu, HE Xiaoman,et al.A cleaner and efficient extraction of Mn from low-grade Mn carbonate ores by ball milling-enhanced Fe2(SO4)3 leaching:Acid consumption reduction[J].Cleaner Engineering and Technology,2021,4:100220.
|
| 8 |
XU Fuyuan, JIANG Linhua, DAN Zhigang,et al.Water balance analysis and wastewater recycling investigation in electrolytic manganese industry of China:A case study[J].Hydrometallurgy,2014,149:12-22.
|
| 9 |
张周位,陈文祥,黄苑龄,等.贵州某低品位碳酸锰矿工艺矿物学及选矿试验研究[J].矿产综合利用,2018(3):66-69.
|
|
ZHANG Zhouwei, CHEN Wenxiang, HUANG Yuanling,et al.Experimental study on process mineralogy and mineral processing of a low-grade manganese carbonate ore in Guizhou[J].Multipurpose Utilization of Mineral Resources,2018(3):66-69.
|
| 10 |
代典,梁欢,何东升,等.湘西地区微细粒级难选菱锰矿浮选试验研究[J].矿产综合利用,2020(4):76-81.
|
|
DAI Dian, LIANG Huan, HE Dongsheng,et al.Experimental study on the flotation of a micro-grained refractory rhodochrosite in western Hunan Area[J].Multipurpose Utilization of Mineral Resources,2020(4):76-81.
|
| 11 |
常征.锰三角地区电解锰压滤渣高温可控脱硫生产活性微粉项目经济效益与社会效益分析[J].地质与勘探,2020,56(4):845-851.
|
|
CHANG Zheng.Economic and social benefits of the project of producing active micropowder from electrolytic manganese slag through high-temperature desulfurization and activation in manganese triangle area[J].Geology and Exploration,2020,56(4):845-851.
|
| 12 |
SONG K,BAE J, LEE G.Determination of Mn oxidation state in Mn-(hydr)oxides using X-ray photoelectron spectroscopy (XPS)[J].2009,42(5),91635473.
|
| 13 |
PHILIPP T, HUITTINEN N, AZZAM S S A,et al.Effect of Ca(Ⅱ) on U(Ⅵ) and Np(Ⅵ) retention on Ca-bentonite and clay minerals at hyperalkaline conditions-New insights from batch sorption experiments and luminescence spectroscopy[J].The Science of the Total Environment,2022,842:156837.
|
| 14 |
杜沛佞,罗仕忠,谭仁俊,等.钇掺杂三氧化二铁催化剂的脱硝性能研究[J].无机盐工业,2022,54(11):137-142.
|
|
DU Peining, LUO Shizhong, TAN Renjun,et al.Study on denitration performance of Y doped Fe2O3 catalyst[J].Inorganic Chemicals Industry,2022,54(11):137-142.
|
| 15 |
李少平.菱锰矿与钙镁碳酸盐矿物晶体结构、表面特性和浮选行为研究[D].赣州:江西理工大学,2019.
|
|
LI Shaoping.Study on the crystal chemistry,surface characteristics and flotation behavior between rhodochrosite and calcium-magnesium carbonate minerals[D].Ganzhou:Jiangxi University of Science and Technology,2019.
|
| 16 |
何静,李崇瑛,张清雨.碳酸盐岩的矿物单体解离研究进展[J].辽宁化工,2021,50(9):1311-1314.
|
|
HE Jing, LI Chongying, ZHANG Qingyu.Research progress in the dissociation of mineral monomers in carbonate rocks[J].Liaoning Chemical Industry,2021,50(9):1311-1314.
|
| 17 |
王帆,田英良.热重-差热分析仪器验证方法与实践[J].玻璃搪瓷与眼镜,2020,48(6):1-7.
|
|
WANG Fan, TIAN Yingliang.Verification and practice of thermogravimetry-differential thermal analyzor[J].Glass Enamel & Ophthalmic Optics,2020,48(6):1-7.
|
| 18 |
邹飞,黄春荣,蹇攀,等.中空球形碳酸锰合成及热分解制备MnOx研究[J].矿冶工程,2021,41(4):156-160.
|
|
ZOU Fei, HUANG Chunrong, JIAN Pan,et al.Synthesis of hollow spherical manganese carbonate and its thermal decomposition for preparation of MnOx[J].Mining and Metallurgical Engineering,2021,41(4):156-160.
|
| 19 |
周强,武斌,陈葵,等.磷尾矿热分解动力学机理与煅烧工艺研究[J].无机盐工业,2023,55(3):47-54.
|
|
ZHOU Qiang, WU Bin, CHEN Kui,et al.Study on thermal decomposition kinetic mechanism and calcination process of phosphorus tailings[J].Inorganic Chemicals Industry,2023,55(3):47-54.
|
| 20 |
马玉文,冯雅丽,李浩然.硫酸锰室温固相球磨制备四氧化三锰[J].无机盐工业,2013,45(2):17-19.
|
|
MA Yuwen, FENG Yali, LI Haoran.Preparation of Mn3O4from manganese sulfate by solid-state ball milling reaction at room tem-perature[J].Inorganic Chemicals Industry,2013,45(2):17-19.
|
| 21 |
吉国荣.添加剂对固相合成镁铝尖晶石材料的影响[D].太原:太原科技大学,2020.
|
|
JI Guorong.Effect of additives on solid phase synthesis of magnesium-alumina spinel materials[D].Taiyuan:Taiyuan University of Science and Technology,2020.
|
 ), FU Chengbing1,2(
), FU Chengbing1,2( ), HU Ping4, YANG Kaixu1,2, CAO Jianxin1,3(
), HU Ping4, YANG Kaixu1,2, CAO Jianxin1,3( )
)