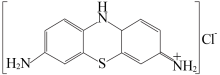
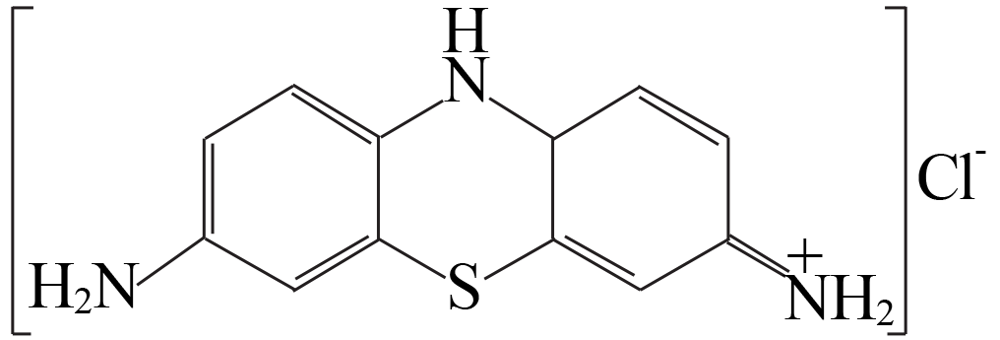
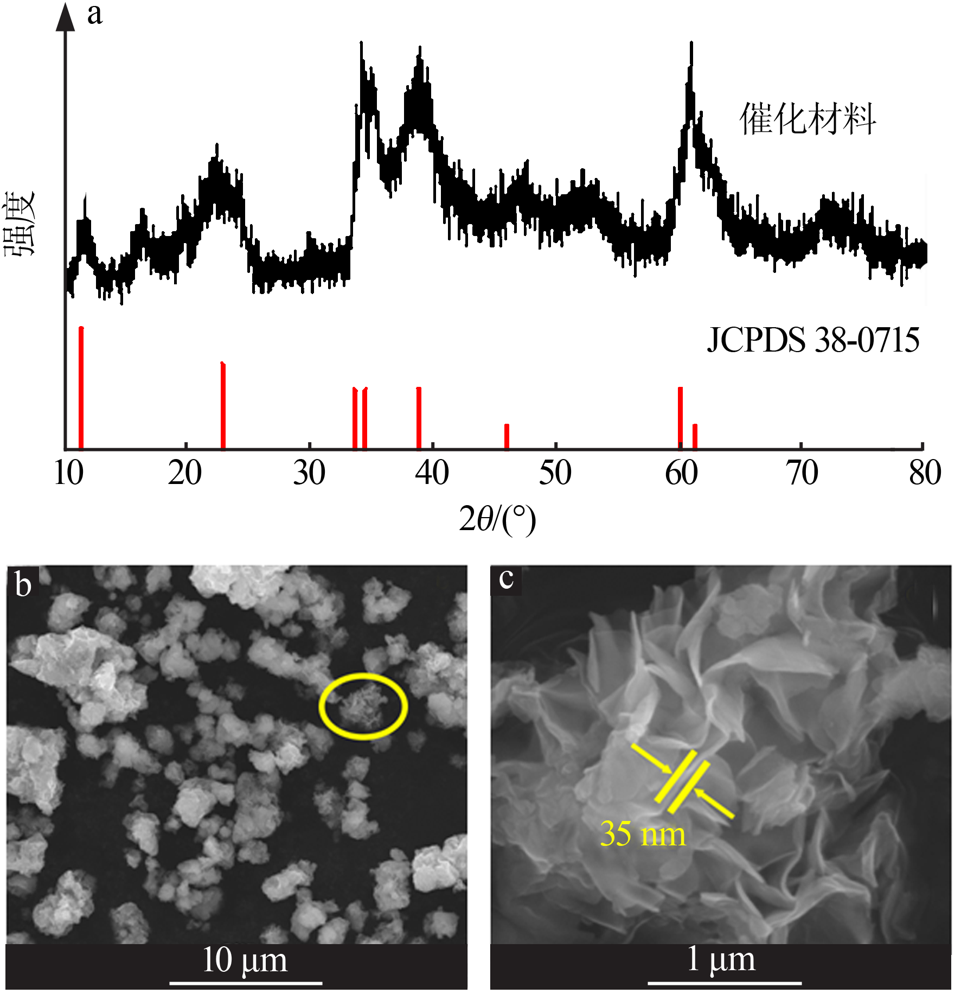
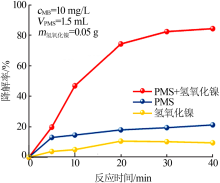
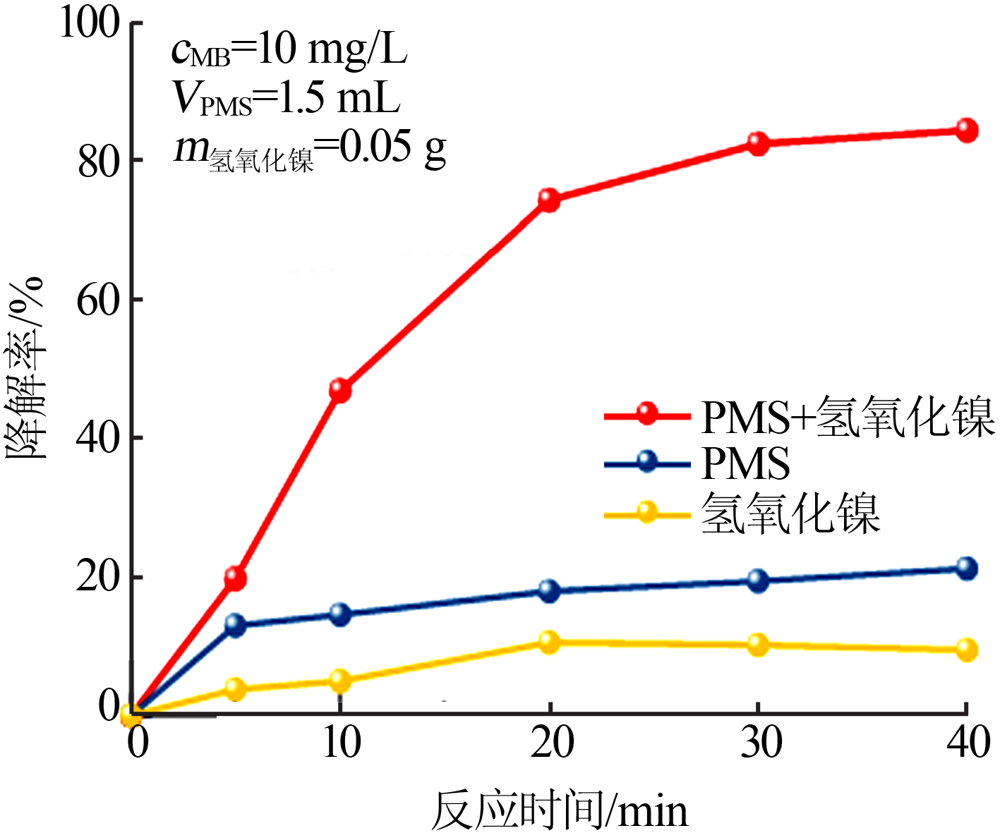
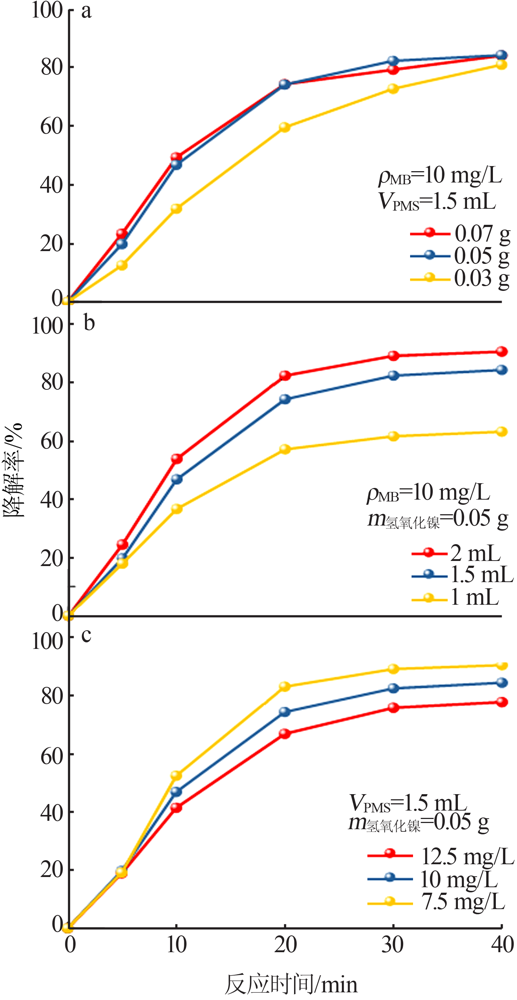
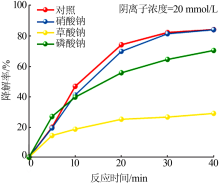
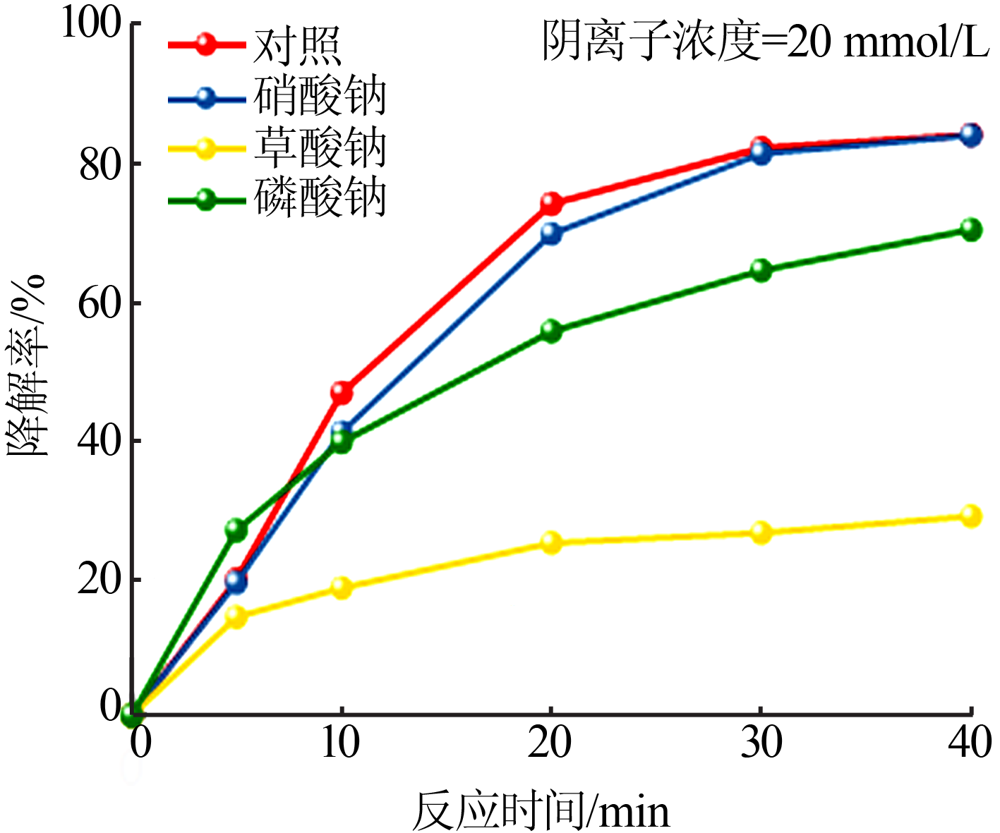
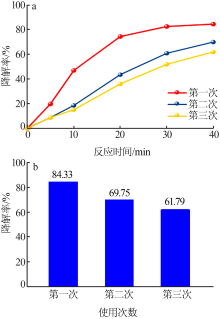
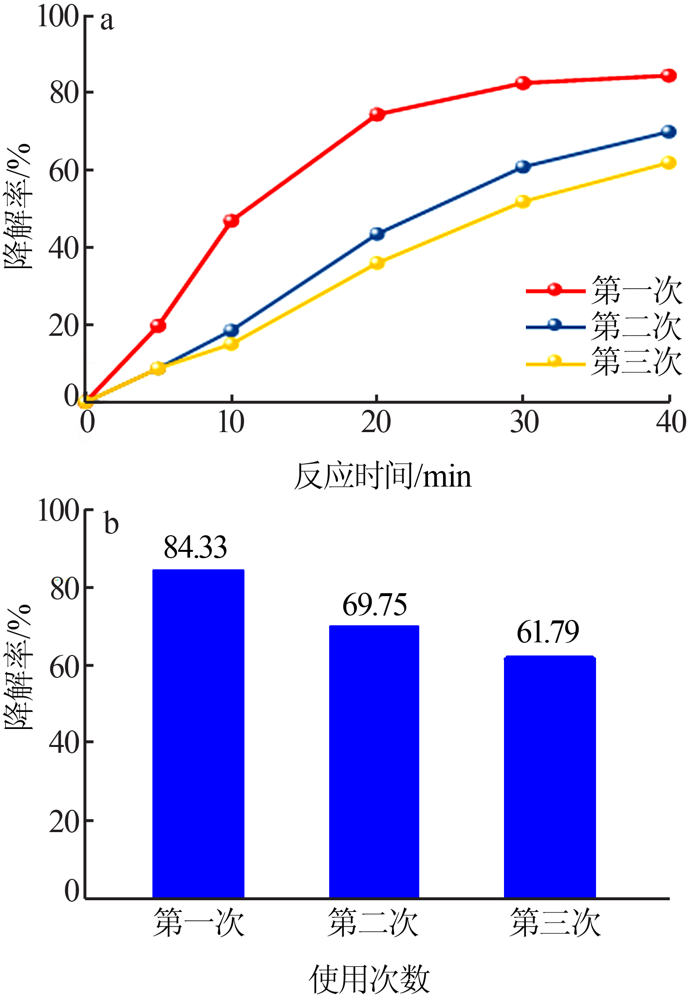
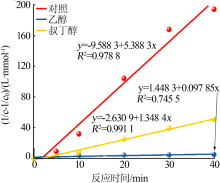
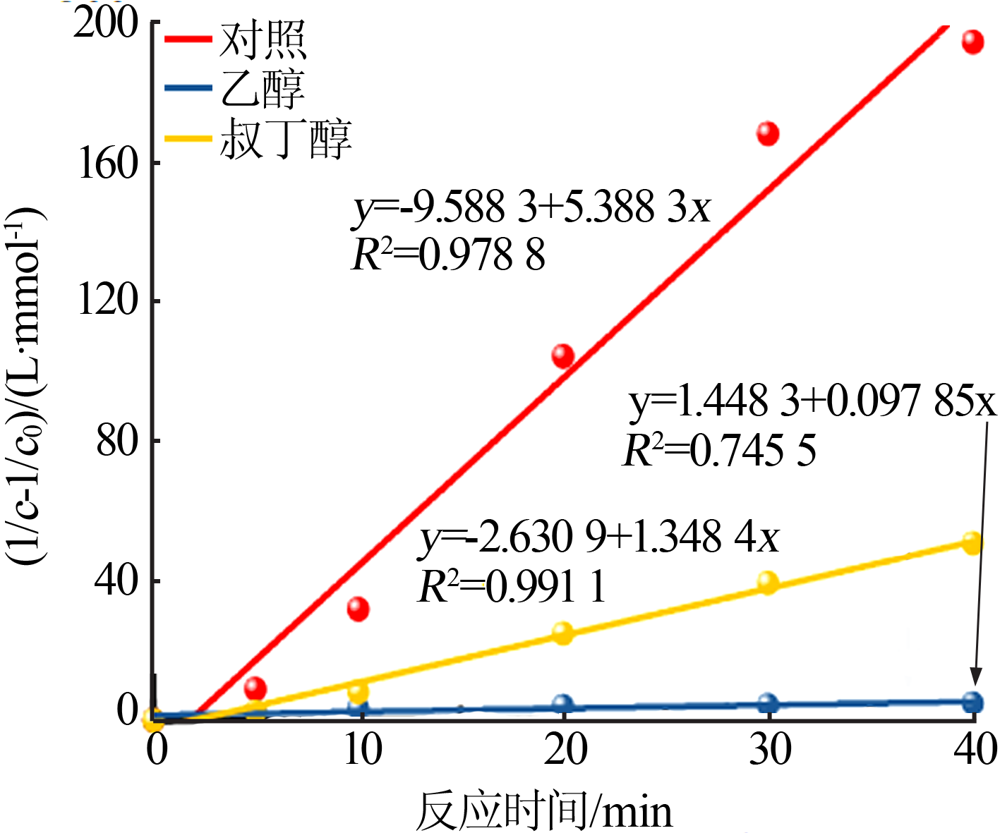
| [1] |
Wang Jianlong, Chen Hai. Catalytic ozonation for water and waste-water treatment:Recent advances and perspective[J]. Science of The Total Environment, 2020, 704.Doi: 10.1016/j.scitotenv.2019.135249.
doi: 10.1016/j.scitotenv.2019.135249
|
| [2] |
Ghauch A, Tuqan A M. Oxidation of bisoprolol in heated persulfate/H2O systems:Kinetics and products[J]. Chemical Engineering Jo-urnal, 2012, 183:162-171.
|
| [3] |
Wang Jianlong, Wang Shizong. Activation of persulfate(PS) and pero-xymonosulfate(PMS) and application for the degradation of emerg-ing contaminants[J]. Chemical Engineering Journal, 2018, 334:1502-1517.
doi: 10.1016/j.cej.2017.11.059
|
| [4] |
施周, 曾寒轩, 邓林. 钴铁镍三元水滑石催化PMS降解水中刚果红[J]. 环境工程学报, 2017, 11(9):4903-4909.
|
| [5] |
汪志. 单过硫酸氢钾复合盐(Oxone)活化技术研究进展[J]. 化学工程与装备, 2019(10):238-240.
|
| [6] |
Wang Jiaqi, Hasaer B, Yang Min, et al. Anaerobically-digested slu-dge disintegration by transition metal ions-activated peroxymono-sulfate(PMS) :Comparison between Co2+,Cu2+,Fe2+ and Mn2+[J]. Science of the Total Environment, 2020, 713.Doi: 10.1016/j.scito-tenv.2020.136530.
doi: 10.1016/j.scito-tenv.2020.136530
|
| [7] |
施周, 衣启航, 卜令君. 镍铁氧体催化过硫酸氢钾对奥卡西平的降解研究[J]. 湖南大学学报:自然科学版, 2016, 43(6):124-129.
|
| [8] |
Liu Yangxian, Wang Yan, Wang Qian, et al. Simultaneous removal of NO and SO2 using vacuum ultraviolet light(VUV)/heat/peroxy-monosulfate(PMS)[J]. Chemosphere, 2018, 190:431-441.
doi: S0045-6535(17)31592-8
pmid: 29024887
|
| [9] |
周骏, 肖九花, 肖冬雪, 等. 热/可见光联合活化单过氧硫酸氢盐光敏降解罗丹明B[J]. 应用化工, 2015, 44(3):397-400.
|
| [10] |
翟俊, 柳沛松, 赵聚姣. 过一硫酸盐碱催化处理染料废水[J]. 中国环境科学, 2020, 40(2):647-652.
|
| [11] |
Farshid Ghanbari, Masoumeh Khatebasreh, Mostafa Mahdavianpo-ur, et al. Oxidative removal of benzotriazole using peroxymonosul-fate/ozone/ultrasound:Synergy,optimization,degradation inter-mediates and utilizing for real wastewater[J]. Chemosphere, 2020, 244.Doi: 10.1016/j.chemosphere.2019.125326.
doi: 10.1016/j.chemosphere.2019.125326
|
| [12] |
张笑丛, 王琮, 吴松海. 不同形貌氧化钴活化过一硫酸盐降解硝基酚[J]. 化学工业与工程, 2020, 37(6).Doi: 10.13353/j.issn.1004.9533.20200112.
doi: 10.13353/j.issn.1004.9533.20200112
|
| [13] |
Jian Songzan, Sun Shengrui, Zeng Yi, et al. Highly efficient persul-fate oxidation process activated with NiO nanosheets with domina-ntly exposed{110} reactive facets for degradation of RhB[J]. App-lied Surface Science, 2020, 505.Doi: 10.1016/j.apsusc.2019.1443318.
doi: 10.1016/j.apsusc.2019.1443318
|
| [14] |
Yan Pengwei, Shen Jimin, Wang Shuyu, et al. Removal of 2,6-di-chlorophenol in water by CuO activated peroxymonosulfate:Effici-ency,mechanism and degradation pathway[J]. Separation and Puri-fication Technology, 2021, 254.Doi: 10.1016/j.seppur.2020.117630.
doi: 10.1016/j.seppur.2020.117630
|
| [15] |
Sun Zhiming, Liu Xiaorui, Dong Xiongbo, et al. Synergistic activa-tion of peroxymonosulfate via in situ growth FeCo2O4 nanoparticles on natural rectorite:Role of transition metal ions and hydroxyl groups[J]. Chemosphere, 2021, 263.Doi: 10.1016/j.chemosphere.2020.127965.
doi: 10.1016/j.chemosphere.2020.127965
|
| [16] |
Tian Xike, Tian Chen, Nie Yulun, et al. Controlled synjournal of dan-delion-like NiCo2O4 microspheres and their catalytic performance for peroxymonosulfate activation in humic acid degradation[J]. Chemical Engineering Journal, 2018, 331:144-151.
doi: 10.1016/j.cej.2017.08.115
|
| [17] |
张明明, 李静, 龚焱, 等. 铁酸锰纳米球修饰石墨相氮化碳光催化活化过一硫酸盐去除双酚A[J]. 环境工程学报, 2019, 13(1):9-19.
|
| [18] |
Kosmulski Marek. Compilation of PZC and IEP of sparingly solu-ble metal oxides and hydroxides from literature[J]. Advances in Co-lloid and Interface Science, 2009, 152(1/2):14-25.
|
| [19] |
曾琪静, 文方, 杨静. 高级氧化技术降解双酚A研究进展[J]. 应用化工, 2018, 47(11):2500-2504.
|
 ),Liao Xiaogang,Li Gang(
),Liao Xiaogang,Li Gang( )
)